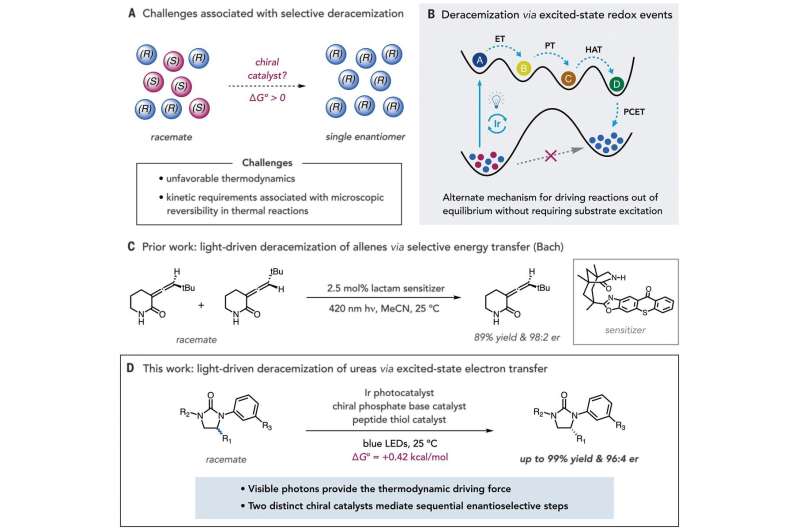
Reaction development. (A) Thermodynamic and kinetic challenges in developing methods for selective deracemization. (B) General, light-driven strategies for achieving out-of-equilibrium deracemization through excited-state redox events. (C) Bach’s report on light-driven deracemization through selective energy transfer. (D) Light-driven deracemization of cyclic ureas by excited-state electron transfer. Credit: Science (2019). DOI: 10.1126/science.aay2204
A combined team of researchers from Princeton University and Yale University has developed a multicatalytic technique to enrich racemic mixtures directly to a single enantiomer in a single step. In their paper published in the journal Science, the group describes their technique, how well it works and the ways it might be used. Alison Wendlandt with the Massachusetts Institute of Technology has published a Perspective piece in the same journal issue discussing the new technique and its possible uses.
In chemistry, deracemization is a process that involves converting a racemate (in which there are equal amounts of right- and left-handed enantiomers of a chiral molecule) to a pure enantiomer. Enantiomers are members of a pair of molecules that are mirror images of one another. Chemists use asymmetric catalysis as a way to make use of just one enantiomer in a pair in certain chemical reactions. But there are times when there is already a desired compound, but it is trapped in a mixture of enantiomers, making it difficult to use. In this new effort, the researchers have developed a technique for handling such a scenario.
The new technique involved using a complementary photochemical catalytic deracemization method to get the job done in just one step. And the researchers found that rather than using an energy-transferring approach, they could use an iridium photocatalyst that promoted reversible electron transfer from the material used as a substrate. They found that the light-induced method triggered a desired succession of proton and hydrogen atom movements. And they noted that both were susceptible to biasing via catalysts that allowed for converting mixtures of cyclic urea enantiomers into just the one that was desired.
Wendlandt notes that the technique involves consuming only photons, unlike other deracemization methods. And the researchers note that selection of the enantiomer is done using two steps that happen in sequence during the catalytic cycle—a means of selection that is better than techniques that involve carrying out the steps individually. They conclude by suggesting their technique represents a new way to create product distributions (that are not in equilibrium) that are between substrate enantiomers.
More information: Nick Y. Shin et al. Light-driven deracemization enabled by excited-state electron transfer, Science (2019). DOI: 10.1126/science.aay2204
Journal information: Science
© 2019 Science X Network