New symmetry-breaking method opens way for bioactive compounds

Many chemical molecules can exist in nature together with their mirror counterparts; like hands, two compounds can be made up of the same atoms in the same overall structure but in opposite orientations, i.e. left-handed and right-handed. This phenomenon of symmetry is called "chirality", and can give mirror counterparts ("enantiomers") entirely different chemical properties. A famous and tragic example of chirality is thalidomide, which was originally sold as a mixture of both enantiomers. The problem was that one was a harmless sedative and the other highly toxic to fetuses, resulting in disturbing congenital deformities.
So today it has become imperative to synthesize compounds with what is known as high "optical purity", which is a measurement of chiral purity: the degree to which a sample contains one enantiomer in greater amounts than the other. But because enantiomers have very small structural differences and identical stability, synthesizing one over the other is a very challenging task.
One way to do this is what chemists call "desymmetrization" of a non-chiral compound that is similar to the target molecule. This involves modifying a molecule so that it loses the symmetry elements that prevented it to be chiral.
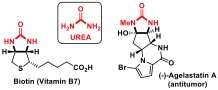
Researchers at Jérôme Waser's Laboratory of Catalysis and Organic Synthesis at EPFL have now developed a new desymmetrization strategy to access chiral building blocks containing urea sub-structures. Urea derivatives are important components of biomolecules such as biotin (vitamin B7) or bioactive natural products, such as the anticancer agelastatin A.
The researchers made two crucial innovations. First, they designed a non-chiral cyclopropane (three-membered carbon ring) precursor. This molecule offers enhanced reactivity and is ideal for reactions under mild conditions.
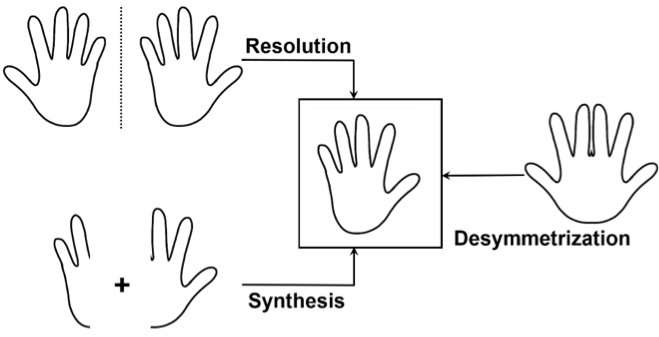
Second, the researchers engineered a new copper catalyst that can form an enantiomer of the desired product with high selectivity. The copper center binds and activates the cyclopropane precursor, causing its bonds to break. The precursor is then attacked by an indole, a molecule very important as structural element of bioactive compounds. As a result, the precursor loses its symmetry - and therefore becomes chiral - and can be used to selectively make the desired enantiomer.
The work is an important breakthrough, as desymmetrization has never been used to access chiral ureas from cyclopropanes before. "New building blocks can be now easily accessed as pure enantiomers, and can be tested for bioactivity or used to synthesize more complex chiral molecules," says Jérôme Waser. "Moreover, the new catalyst we have designed certainly will be useful for other applications in synthetic chemistry."
More information: Daniele Perrotta et al, Lewis Acid Catalyzed Enantioselective Desymmetrization of Donor-Acceptor Meso-Diaminocyclopropanes, Angewandte Chemie International Edition (2018). DOI: 10.1002/anie.201800494
Journal information: Angewandte Chemie International Edition
Provided by Ecole Polytechnique Federale de Lausanne