The multitasking catalyst

Professor Takashi Ooi and his team of researchers from Nagoya University, Japan, have designed a catalyst that performs two tasks during the course of the reaction.
Chirality – the property held by some molecules that means a molecule is non-superimposable on its mirror image – is a key concept in molecular synthesis. We refer to a molecule's mirror-image molecule as its enantiomer.
This matters because enantiomers, despite being so similar, can interact with their environment in completely different ways. For example one may be a therapeutic drug whilst the other could be harmful to human health. A famous and tragic example was the drug thalidomide, which existed in two forms – one cured morning sickness and the other produced deformities in children.
Many chiral molecules have one or more stereocentres – that is an atom with four different substituents. The substituents can be arranged around the central atom in two different ways, giving two entantiomers. If there are two stereocentres there could be four arrangements in total – known as stereoisomers.
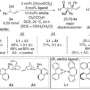
Professor Ooi and his group have developed a method of synthesising complex molecules with specific chiralities. They start with an oxindole and a secondary alkyl bromide, as racemic mixtures (1:1 mixtures of enantiomers). When the two molecules react together they produce a molecule with two stereocentres – and four possible stereoisomers.
They have developed a catalyst that is capable of directing the reaction at both stereocentres simultaneously, selecting for just one stereoisomer out of the possible four.
"We believe that this research provides an efficient way to access complex chiral molecules and would contribute to the discovery of new functional organic molecules, such as pharmaceuticals," he says.
More information: Kohsuke Ohmatsu et al. Diastereo- and enantioselective phase-transfer alkylation of 3-substituted oxindoles with racemic secondary alkyl halides, Chemical Communications (2017). DOI: 10.1039/C7CC07122A
Journal information: Chemical Communications
Provided by Royal Society of Chemistry