Credit: Nagoya University
The research team led by Dr. Daisuke Ono and Prof. Akihiro Yamanaka of the Graduate School of Medicine, Nagoya University, along with collaborators, has revealed that inhibitory neurons (GABAergic neurons) of the central circadian clock in the suprachiasmatic nucleus (SCN) refine circadian output rhythms.
Physiology and behavior such as sleep/wakefulness, body temperature, and endocrine functions, exhibit 24-hour oscillations called circadian rhythms. The temporal order of physiology and behavior is regulated by the central circadian clock located in the SCN. Our findings can be developed to understand how the SCN regulates physiological phenomena. Furthermore, it might aid the development of new clinical approaches for a variety of diseases related to the circadian clock. These achievements were published online in Communications Biology on June 21, 2019.
The temporal order of physiology and behavior in mammals is controlled by the master circadian clock located in the SCN. The SCN generates an endogenous circadian oscillation that entrains a day-night alternation. The SCN is composed of heterogeneous neurons with various neurotransmitters. Of these, an inhibitory neurotransmitter, γ-Amino-Butyric-Acid (GABA) is expressed in almost all SCN neurons; however, its role in circadian physiology is still unclear.
Research results
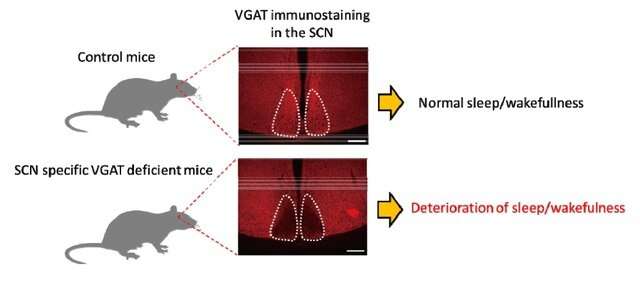
In the present study, we examined GABA signaling in the SCN using mice lacking vesicular GABA transporter (VGAT-/-) or a GABA synthesizing enzyme, glutamate decarboxylase (GAD65-/-/67-/-). We simultaneously measured circadian rhythms with a bioluminescence reporter for the clock gene product PER2 (PER2::LUC), spontaneous firing, and intracellular calcium (Ca2+) levels for several circadian cycles in cultured SCN slices of perinatal mice. SCN lacking GABA exhibited burst firing throughout a day. The burst firing was associated with an abrupt increase in intracellular Ca2+, which was synchronous throughout the entire SCN slice. By contrast, the circadian PER2 rhythm was essentially kept intact. We also found that SCN-specific VGAT depletion in adult mice deteriorated the circadian behavioral rhythms.
Research summary and future perspective
In conclusion, GABA is necessary for suppressing the burst firing of neuronal activity and abrupt increases of intracellular Ca2+ levels but not for the generation and stability of the molecular circadian oscillation in the SCN. The GABA network may refine the circadian firing rhythm to ensure noiseless communications with neurons outside the SCN.
The article, "GABA in the suprachiasmatic nucleus refines circadian output rhythms in mice" has been published online in Communications Biology.
More information: Daisuke Ono et al. GABA in the suprachiasmatic nucleus refines circadian output rhythms in mice, Communications Biology (2019). DOI: 10.1038/s42003-019-0483-6
Journal information: Communications Biology
Provided by Nagoya University