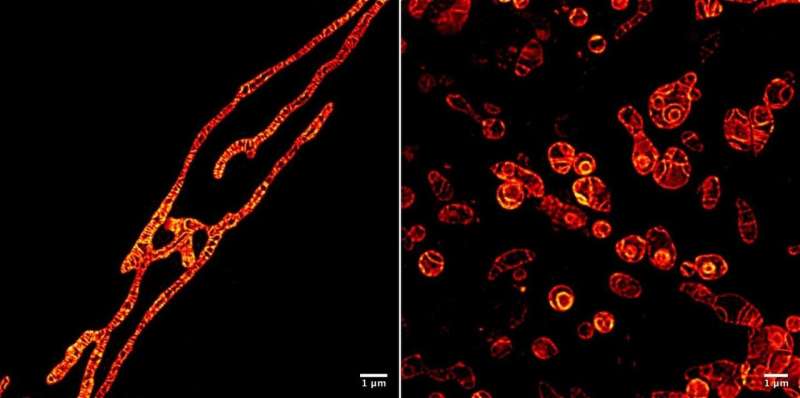
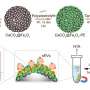
Inner membranes of live mitochondria under a STED microscope imaged using the MitoPB Yellow fluorescent marker molecule created by researchers at the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University. The outer membranes of the mitochondria are invisible. The marker molecule can withstand the STED beam for a relatively long time, which allows time-lapse imaging of the live subject. Sample preparation is much easier for an optical microscope than a Transmission Electron Microscope (TEM), requiring about an hour rather than a day. Cells cannot be imaged alive using TEM. The mitochondria have been treated with a reagent that suppresses DNA replication, inducing dysfunction, in order to see their survival (left) and dying (right) processes. Being able to see the dysfunction processes occurring inside mitochondria will lead to a better way of diagnosing human mitochondrial disease - and perhaps even a cure. Credit: © ITbM, Nagoya University
Light microscopy is the only way in which we can look inside a living cell, or living tissues, in three dimensions. An electron microscope only gives a two-dimensional view, and the organic sample would quickly burn up due to the extreme heat of the electron beam, and therefore cannot be observed alive. Moreover, by marking the biomolecules of the structure we are interested in with a specially designed fluorescent molecule, we can distinguish it from the surroundings: this is fluorescence microscopy.
Until the mid-1990s fluorescence microscopy was hampered by basic physics: due to the diffraction limit, any features on the sample closer together than about 250 nanometres would be blurred together. Viruses and individual proteins are much smaller than this, so they could not be studied this way. But around 1994, in a wonderful lesson teaching us that we must take care when applying fundamental physical principles, Stefan Hell discovered Stimulated Emission Depletion (STED) microscopy, which is now one of several optical microscopy approaches that achieve "super-resolution," resolution beyond the diffraction limit. He received the Nobel Prize in Chemistry in 2014 "for the development of super-resolved fluorescence microscopy," together with Eric Betzig and William Moerner.
To see why the diffraction limit is a problem, imagine the structure of interest is very small, say, 50 nanometres across, like a virus, and has been marked with a fluorescent biomolecule. Now imagine illuminating it with a laser spot, say, 200 nanometres in diameter. The illuminated marker molecules emit light spontaneously, at random times, by fluorescence, with the probability dropping rapidly with time. The photons from many fluorescing molecules are focused onto a detector using lenses, creating a single featureless pixel. It's not fully bright because only a small proportion of the sample in the illuminated circle contains fluorescent molecules. If you were to move the laser 200 nanometres in any direction, to where, in this example, no fluorescent molecules are present, the signal will certainly go dark. So, this rather dim pixel tells us that something is present inside this sample area 200 nanometres in diameter. The diffraction limit prevents us forming pixels from smaller areas, if we use the basic approach.
The physical idea of STED microscopy is very simple. With the laser spot illuminating the region around the small fluorescing structure again, suppose you somehow stop light being sent to the detector from as large an area as possible within the spot—leaving a much smaller spot, say, 60 nanometres in diameter. Now if you move the laser 60 nanometres in any direction and the signal goes dark, the pixel in the image represents the presence of structure up to 60 nanometres across. The diffraction limit has been beaten. Of course, one such pixel is featureless, but a sharp image of mitochondria can be built up by scanning across and recording many pixels of varying brightness. (See Figure 1. "Time-gated STED Microscopy" was used to capture most of the images in this paper.)
Stefan Hell's Nobel Prize-winning discovery consists of two insights. First, he thought of the idea of stopping light being sent to the detector from as large an area as possible within an illuminated spot whose size matches the diffraction limit. Second, he figured out how to actually achieve it.
Two lasers illuminate the same spot. The first laser excites the marker molecule electrons and they decay spontaneously back to their ground state, each emitting a visible photon of a specific wavelength. (This is fluorescence.) The process is random, with the emission probability decreasing with time fairly quickly, meaning that most photons are emitted within the first few nanoseconds of the sample being illuminated. A second laser, the "STED beam," shaped with a hole in the middle so as not to affect the marker molecules there, is tuned to stimulate emission of a photon by the excited marker molecule in the outer ring. But how are these photons distinguished from photons emitted from the middle?
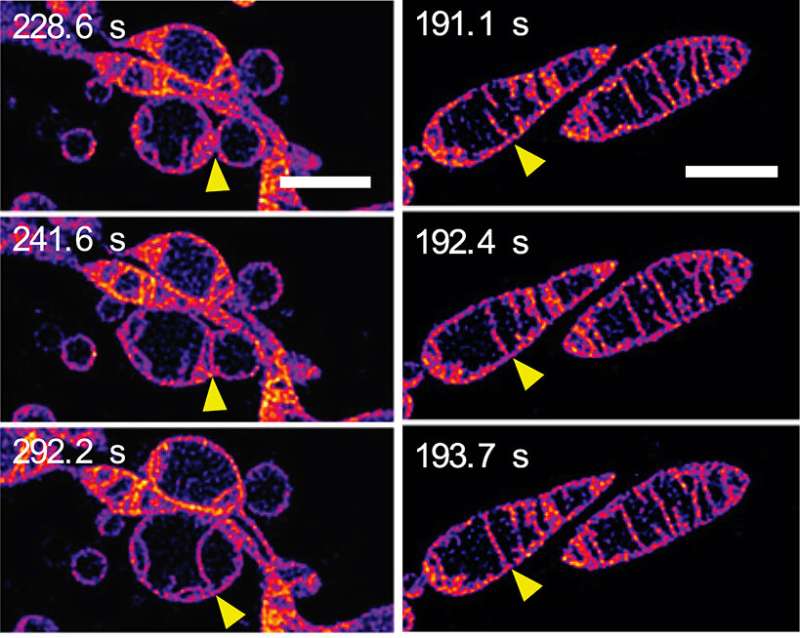
In response to being deprived of nutrients, mitochondria fuse together and increase the number of cristae. (a) Frames from a time-lapse sequence showing two separate mitochondria fusing together to form a single mitochondrion. The outer membranes of the mitochondria are invisible: we are seeing the inner membranes fusing together. (b) Frames from a time-lapse sequence showing two cristae inside a single mitochondrion fusing together. (See Video 2 in the Supplementary Material on the paper's PNAS webpage.) The scale bars represent 2mm. Credit: © ITbM, Nagoya University
The emission process from the outer ring is also random but happens much more quickly, the probability decreasing rapidly, meaning that most of these photons are emitted within a nanosecond or so. As the two superimposed beams scan across the sample, by the time the centre of the ring is fluorescing, the surrounding molecules have already been forced into their ground state by emitting a photon—they have been "switched off." The STED microscopy technique relies on clever timing in this way. In principle, the size of the glowing central spot can be made as small as you want, so any resolution is possible. However, the doughnut-shaped "STED beam" would then be delivering energy in the form of concentrated visible laser light to a larger area of the living cell, risking killing it.
Nevertheless, the process is not ideal, and the resulting image loses some sharpness because some marker molecules in the outer ring are not properly switched off—the process is probabilistic, after all—and when they do fluoresce they contaminate the signal from the centre. However, due to the different timing of the spontaneous and stimulated emission, the earliest photons to arrive at the detector are from regions illuminated by the highest STED beam intensity, and the last photons to arrive are most likely from marker molecules located in the central spot. So by waiting a short time (around one nanosecond) before recording the image, most of the photons from the outer ring can be filtered out. This is called "Time-gated STED Microscopy." Further sharpening of the image is achieved through a process called deconvolution.
The invention of super-resolution microscopy heralded a leap forward in the life sciences. Living organisms could be observed at an unprecedented resolution. However, time-lapse sequences of images could not be made over any decent length of time because the marker molecules would degrade under the intense STED beam and stop fluorescing. This is the photobleaching problem. The damaged marker molecules can also become toxic to the cell.
The photobleaching problem solved
Shigehiro Yamaguchi and Masayasu Taki, of Nagoya University's Institute for Transformative Bio-Molecules (ITbM), led a research team that has developed a marker molecule, called "MitoPB Yellow," that is absorbed by the inner membrane of mitochondria, including the cristae—the fold-like structures—and has a long lifetime under a STED beam. The idea for the marker molecule targeting mitochondria came from co-author Chenguang Wang, of the ITbM. Multicolour STED imaging with a single STED laser is also possible; and the researchers expect that fluorescent markers similar to MitoPB Yellow should find a wide range of applications in other super-resolution techniques as well (such as those developed by Eric Betzig and William Moerner).
To demonstrate the practical usefulness of MitoPB Yellow for live-cell imaging, the group placed mitochondria under conditions that are known to cause certain structural changes—but until now these have only been observed using transmission electron microscopy, which cannot be used on live cells. The mitochondria were treated with a reagent that suppresses DNA replication, inducing dysfunction, in order to observe their survival and dying processes.
Video 1. Live mitochondria imaged in unprecedented detail -- for an unprecedented length of time -- using the MitoPB Yellow fluorescent marker created by Nagoya University-led researchers. The marker molecule is designed to be absorbed by only certain membranes within each mitochondrion, and retains its fluoresescence under the STED microscope for a very long time. This video was shot at 1.5 fps and a resolution of 90nm. Still images were captured at 60nm resolution. In response to being deprived of nutrients, mitochondria fuse together and increase the number of cristae. This time-lapse sequence shows events such as two separate mitochondria fusing together to form a single mitochondrion; and a single mitochondrium fusing together. Note that the outer membranes of the mitochondria are invisible: we are seeing the inner membranes fusing together. Credit: ITbM, Nagoya University
Then, using Time-gated STED Microscopy, the research team made still images at 60 nanometre resolution (about one thousandth of the width of a human hair), as well as time-lapse image sequences showing the mitochondria responding to a deprivation of nutrients by changing form in order to survive. The long image sequences—of up to 600 images—are the first ever made of mitochondria at the relatively high spatial resolution of 90 nanometres. (See Video 1, which shows a time-lapse sequence recorded over nearly 7 minutes.)
Over a few minutes the inner mitochondrial structure changed dramatically in a number of ways. Initially, elongation and increase in the number of cristae was seen. One image sequence (see Figure 2a) shows inner membranes of neighbouring mitochondria fusing together—in other words, two mitochondria fusing to make one. Another image sequence (see Figure 2b) shows two cristae within a single mitochondrion apparently fusing together. Elongation and creating more cristae is thought to increase the efficiency of energy production (ATP synthesis) while protecting the mitochondrium from "autophagosomal degradation"—a programmed death whose purpose is to remove unnecessary or dysfunctional components from the cell and allow the orderly degradation and recycling of cellular components.
After the initial period of elongation, the inner membranes of some mitochondria split into globules that swelled and lost cristae (see Movie S2); some globules ruptured (Movie S4). Some formed concentric spheres (Figure 1 and Video 1). The fluorescence intensity remained the same. Noteworthy here is that the cristae and membranes remain as sharply imaged as before, which indicates that the cause of the mitochondrion's death is not toxicity due to degradation of the marker molecule under the beam. The extremely strong STED laser might have damaged the mitochondria, although exactly why they rupture is unknown.
In these images, after seeing initial survival responses, we are watching the death of mitochondria under the intense STED beam. A future direction of research will be to reduce the intensity of the STED laser beam by creating a fluorescent marker molecule that glows when illuminated by light of a longer wavelength and therefore lower energy. The mitochondria might then live longer.
However, even with MitoPB Yellow, the dying process—which is not well understood—can be studied. Nobody knows if the morphological (structural) changes observed during the dying process are related to apoptosis (normal, controlled death) or necrosis (death due to injury or malfunction). Apoptosis is known to be triggered by a signalling molecule called cytochrome C: if a reagent can be found that suppresses cytochrome C, then mitochondria—and human cells—could live longer.
Being able to see the processes occurring inside mitochondria should lead to a better way of diagnosing human mitochondrial disease—and perhaps even to a cure.
More information: Chenguang Wang et al, A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae, Proceedings of the National Academy of Sciences (2019). DOI: 10.1073/pnas.1905924116
Journal information: Proceedings of the National Academy of Sciences
Provided by Nagoya University