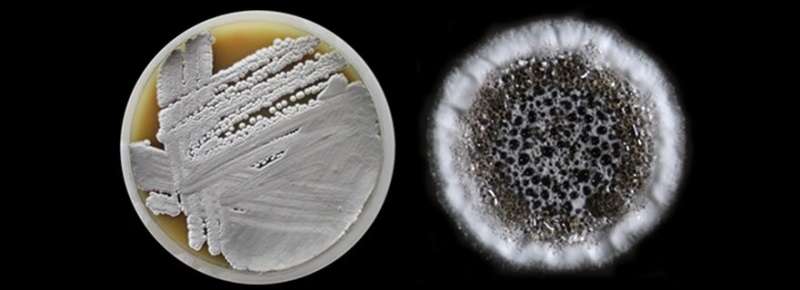
Nutritional soil with a producer strain of Streptomyces together with the inclusion of a single colony. Credit: Leiden University
Increasing resistance and a lack of new antibiotics are a serious problem for public health. Against this background, Gilles van Wezel of the Institute of Biology Leiden is looking for new medicines. Together with former Ph.D. student Changsheng Wu and colleagues he discovered the special antibiotic lugdunomycin, which they named after Leiden. The discovery was recently published in the journal Angewandte Chemie.
Exhausted source
"It is predicted that by 2050 approximately 10 million people worldwide will die from the consequences of antibiotic resistance," says Van Wezel. "That is as many as due to cancer. We are facing a negative trend in which more and more bacteria are becoming resistant, often to multiple antibiotics, while at the same time fewer and fewer new medicines are coming onto the market. We want to find solutions for this." Van Wezel performs research into the bacterium Streptomyces. This genus lives in the soil and produces no less than two-thirds of all antibiotics. Because they have been used and screened for so long, the pharmaceutical industry thought that everything that could be obtained from these bacteria had already been discovered. The source seemed exhausted," says Van Wezel. But that turned out not to be the case after all.
Sleeping antibiotic
At the beginning of the 21st century, DNA sequencing took off: a technique for visualising and analysing the entire DNA of organisms. "This way we could also look at the DNA of antibiotic-producing bacteria. Then it turned out that they had much more potential than we had previously thought possible! The Streptomyces coelicolor model strain, which produces sky blue (coelicolor in Latin) antibiotics, is a good example. The bacterium produces as many as four different species. Thousands of scientists have been working on this strain for fifty years, so it was thought that there was not much more to be obtained. However, when the complete DNA of the strain was published in 2002, it appeared that the DNA of the bacteria contains far more recipes for antibiotics than just for the antibiotics they produce in the lab. These are called sleeping antibiotics.
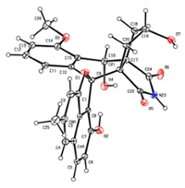
The structure of the antibiotic lugdunomycine. Credit: Leiden University
Saving energy
It takes a bacterium a lot of energy to make antibiotics," explains Van Wezel. "That's why they don't do it when it's not necessary." Therefore, in the laboratory bacteria make fewer antibiotics than in the soil. Simply because the lab lacks the necessary ecological conditions. "Or, in other words: in the soil, there are more enemies against which bacteria need antibiotics."
Leiden antibiotic
The antibiotic discovered by Wu, Van Wezel and co-promoter Young Hae Choi is also a sleeping antibiotic, produced by a still unknown Streptomyces bacterium from the Qinling Mountains in China. They called it lugdunomycin, after the Latin name for Leiden (Lugdunum batavorum). "Wu has looked at a strain that did not produce anything at first sight. But after imitating different growing conditions, we nevertheless witnessed biological activity here. This led to the discovery of a chemical molecule with an unforeseen complex structure!" Lugdunomycin is derived from a well-known family of molecules with mainly anti-tumour activity but underwent such large modifications that it no longer looks like it. "The addition of three extra rings make it look like a helicopter," says Van Wezel. "We have never seen this before, but it will certainly occur more often in nature. But in such small quantities that they must have been overlooked until now."
The discovery of such a radically different chemical structure is rare. Now that Wu, Choi and Van Wezel have published the structure of lugdunomycin, the next challenge is: to make Streptomyces produce more of it. They also have to investigate the exact activity of the molecule, and whether it is actually clinically applicable. Possibly, the Leiden antibiotic will be able to serve as real medicine in the future. To this end, Van Wezel started a follow-up project within the NACTAR programme in the NWO domain of Applied and Technical Sciences (TTW). In this programme, scientists and industry work together on the development of new antibiotics. "For now, at least, we have discovered this special molecule, which is very exciting. I don't think we will quickly discover another molecule with such a spectacular structure."
More information: Changsheng Wu et al. Lugdunomycin, an Angucycline-Derived Molecule with Unprecedented Chemical Architecture, Angewandte Chemie International Edition (2019). DOI: 10.1002/anie.201814581
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Leiden University