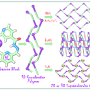
Synthesis scheme of 7-oxa-2-azabicyclo[2.2.1]hept-5-ene and its structure. Credit: Fedor Zubkov
Researchers from the People's Friendship University of Russia (RUDN University) have refined our understanding of the structure of synthetic toxins that impede the development of red lionfish embryos, but in their modified form can be used for studying embryos of vertebrata. The results are published in Organic and Biomolecular Chemistry.
"A RUDN alumnus and my teacher, Vladimir Kouznetsov, also a chemistry professor, who works in Columbia now, had a paper published in the same journal in 2013. He described the synthesis of a previously unknown heterocyclic system of 7-oxa-2-azabicyclo[2.2.1]hept-5-ene. During one of the stages, from simple compounds, furfurylamines and maleimides, a complex skeletal molecule with prominent biological features was formed. For example, it could be used as an inhibitor for early stages of embryonic development of vertebrata," relates one of the article's authors, Ph. D., Fedor Zubkov, associate professor at the Department of Organic Chemistry, RUDN University.
But when re-conducting the experiment, the researchers discovered that a different compound is formed. It turned out that the system of 7-oxa-2-azabicyclo[2.2.1]hept-5-ene, described in Vladimir Kouznetsov's article, has spectral properties very similar to another class of organic compounds, 3-(furylmethylamino)-N-R-pyrrolidine-2,5-diones, which are, in fact, the products of the described reaction.
Using the combined data of X-ray diffraction analysis and nuclear magnetic resonance method (NMR), the researchers unambiguously determined the spatial structure of the reaction product. The mistake was in incorrect interpretation of the NMR data in the paper of 2013. The researchers from RUDN invited Vladimir Kouznetsov, the author of the 2013 paper, to contribute to their article refuting the previous one, in which they have explained the causes for differing results of the synthesis in two work groups.
Carbon and hydrogen are parts of every organic compound, so NMR spectroscopy is the best method for analyzing them, giving the most detailed information on the molecule's spatial structure. The NMR method of research means that the sample is placed in a strong magnetic field and exposed to high-frequency radiation, the frequency of which is, in certain points, equal to the absorption frequency of an atomic nucleus (mostly the nuclei of carbon and hydrogen are studied). If the setting of atoms in the molecule differs, their nuclei absorb energy in different frequencies, which is seen in their spectra as absorption signals. These signals' location and shape help to determine the quantity and type of certain nuclei in the molecule. After further mathematic and analytic work, it becomes possible to fully interpret the structure of the molecule: the spectra "show" the position of every atom in the compound. To verify the data from the NMR method, the scientists used X-ray diffraction analysis, through which it is possible to take a photograph of the molecule and to prove its structure unambiguously.
"We have determined the chemical environment of the carbon and hydrogen nuclei that are in the molecule, and our interpretation did not match with the interpretation that professor Kouznetsov's group suggested earlier. This uncertainty prompted us to conduct a more detailed study," Fedor Zubkov says.
More information: F. I. Zubkov et al, Comment on "An unexpected formation of the novel 7-oxa-2-azabicyclo[2.2.1]hept-5-ene skeleton during the reaction of furfurylamine with maleimides and their bioprospection using a zebrafish embryo model" by C. E. Puerto Galvis and V. V. Kouznetsov, Org. Biomol. Chem., 2013, 11, 407, Org. Biomol. Chem. (2017). DOI: 10.1039/C7OB01207A
Provided by RUDN University
![Synthesis scheme of 7-oxa-2-azabicyclo[2.2.1]hept-5-ene and its structure. Credit: Fedor Zubkov RUDN chemists identified the structure of the agent causing mutations in lionfish embryos](https://scx1.b-cdn.net/csz/news/800a/2017/2-rudnchemists.jpg)