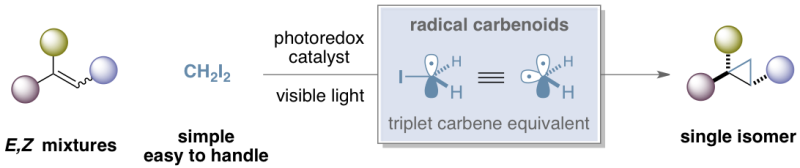
The 'radical carbenoid', discovered at ICIQ in Tarragona, allows stereoconvergent cyclopropanation reaction. Credit: © ICIQ / © Wiley-VCH
Today, chemists synthesize cyclopropanes using pure E or Z alkenes as starting materials and usually dangerous, unstable reagents such as diazomethane or iodomethylzinc iodide.
Now, a team of researchers led by Dr. Suero at the Institute of Chemical Research of Catalonia in Tarragona (Spain) has designed a new strategy that allows the stereoconvergent preparation of trans-cyclopropanes starting from E/Z alkene mixtures. Moreover, this photoredox catalytic method uses diiodomethane—a commercially available, easily handled reagent—as the methylene source.
This research represents the first example of a stereoconvergent cyclopropanation reaction. It is also the first proposal of a new type of carbenoid species called 'radical carbenoids,' theoretically equivalent to a triplet carbene but with a completely unprecedented reactivity.
More information: Ana M. del Hoyo et al, A Stereoconvergent Cyclopropanation Reaction of Styrenes, Angewandte Chemie International Edition (2016). DOI: 10.1002/anie.201610924
Journal information: Angewandte Chemie International Edition
Provided by Institute of Chemical Research of Catalonia (ICIQ)