Credit: Toshiba Corporation
Toshiba has achieved an advance in photo-electrochemical processing that converts carbon dioxide directly into ethylene glycol with an efficiency of 0.48%. Ethylene glycol is a useful and versatile industrial raw material that can be used to manufacture polyester fibers, PET bottles and antifreeze formulations.
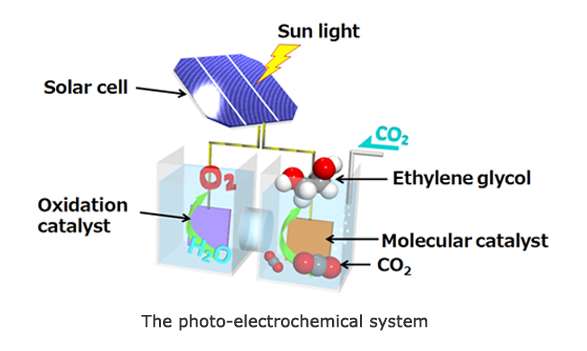
The high conversion rate is due to Toshiba's development of a silicon-based photovoltaic cell as the electricity source for a molecular catalyst to electrochemically reduce carbon dioxide in an aqueous solution and convert it into ethylene glycol. The technology was presented at the PRiME 2016 international conference held in Honolulu, Hawaii, on October 2-7, 2016.
Rising concentrations of carbon dioxide in the atmosphere are recognized by the International Panel on Climate Change and other expert bodies as a primary cause of climate change, and this, along with growing concerns for fossil fuels, is stimulating efforts to develop renewable and low-carbon energy sources. In parallel, efforts are also being made around the world to develop photo-electrochemical cell technology for converting CO2 into chemical energy, as a countermeasure against both climate change and fossil-fuel concerns.
Previously reported electrochemical catalyst have converted carbon dioxide into two-electron reduction products, such as carbon monoxide and formic acid, stimulating a search for catalysts that support more complex reduction reactions and produce a multi-electron reduction substances. This approach is seen in the use of a copper catalyst to trigger direct conversion into hydrocarbons. However, this approach also generates a large quantity of by-products, a problem in itself.
Credit: Toshiba Corporation
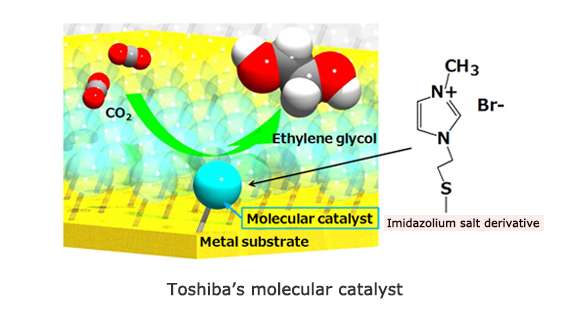
Toshiba's work in molecular catalysts has led to the development of a photo-electrochemical system (patent pending) that uses a silicon-based photovoltaic cell as the source of electricity for molecular catalysts to electrochemically reduce carbon dioxide into ethylene glycol. Conversion to ethylene glycol using the photo-electrochemical system produces an energy conversion efficiency of 0.48%.
The newly developed catalyst is an imidazolium salt derivative adsorbed at high density onto a metal surface. When carbon dioxide molecules interact with the derivative, a reaction not previously achieved occurs, a result attributed to the molecular catalyst promoting both a direct reaction with the carbon dioxide and serving as a reaction field for multi-electron reduction reactions.
The ethylene glycol generated by the system is a valuable and versatile industrial raw material that can be used in the manufacture of PET bottles, polyester fibers and resins. Toshiba will continue to develop the technology, targeting commercialization of a highly efficient system for producing industrial raw materials by the 2020s.
Provided by Toshiba Corporation