Credit: ITbM, Nagoya University
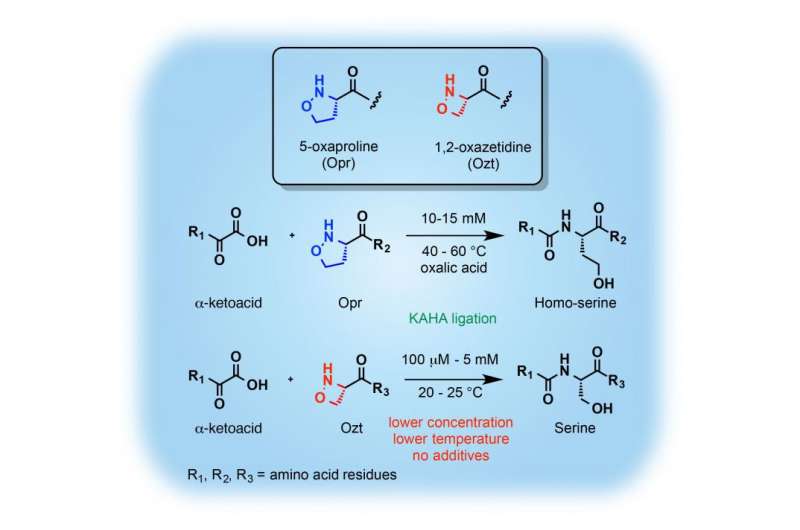
The development of new methods for the chemical synthesis of proteins is highly significant to access a range of proteins inaccessible by conventional approaches. Dr. Ivano Pusterla and Prof. Jeffery Bode of ETH-Zürich and Nagoya University's Institute of Transformative Bio-Molecules (ITbM) have succeeded in the first synthesis of oxazetidine amino acids as a new ligation partner for the rapid and chemoselective synthesis of proteins.
Oxazetidine is a hydroxylamine in a four-membered ring form and exhibits high reactivity arising from its ring strain. The KAHA ligation reaction developed by Bode's group has been used to synthesize various proteins by the reaction between α-ketoacids and oxaprolines, a five-membered hydroxylamine ring. One limitation of this previous reaction system was that a non-native homoserine residue is introduced in the ligation site. In this study, Bode has showed that oxazetidine-containing peptides react with α-ketoacids to undergo KAHA ligation at lower concentrations and at milder temperatures to produce proteins that contain native serine residues. The oxazetidine amino acid is formally an oxidized form of serine, which has a relatively high abundance and can replace other amino acid residues without affecting the overall folding or function of proteins.
This reaction system was applied towards the synthesis of a 100-residue calcium-binding protein, S100A4, which is usually difficult to access by native chemical ligation or other amide-forming reactions. The high reaction rate, chemoselectivity and versatility of this new native amide-forming ligation reaction using oxazetidine amino acids is envisaged to become a powerful method for the rapid chemical synthesis of useful proteins.
More information: An oxazetidine amino acid for chemical protein synthesis by rapid, serine-forming ligations, Nature Chemistry (2015) DOI: 10.1038/nchem.2282
Journal information: Nature Chemistry