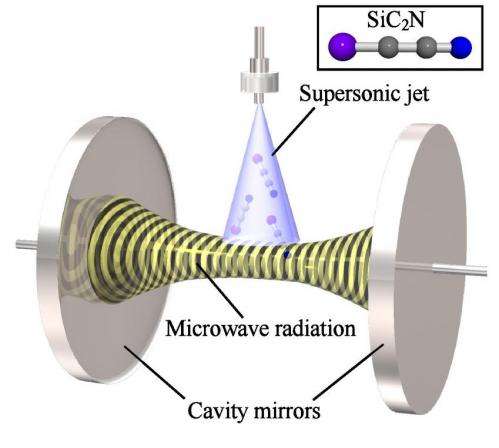
Schematic figure of the present experiment. Reactive molecules produced in a supersonic jet come into the microwave cavity placed inside a vacuum chamber, where microwave radiation excites the molecules. Induced microwave radiation from the excited molecules is detected. Credit: Yasuki Endo/ The University of Tokyo
Silicon, which is one of the most common elements in the Earth's crust, is also sprinkled abundantly throughout interstellar space. The only way to identify silicon-containing molecules in the far corners of the cosmos - and to understand the chemistry that created them - is to observe through telescopes the electromagnetic radiation the molecules emit.
Scientists from the University of Tokyo, in Japan, have now determined the unique electromagnetic emission spectrums of two new, highly-reactive silicon compounds. The research, which is published in The Journal of Chemical Physics from AIP Publishing, will help astronomers look for the molecules in the interstellar medium.
"Like human fingerprints and DNA sequences are the markers of human identity, we can identify molecules from the frequencies of the electromagnetic waves emitted by them," said Yasuki Endo, a researcher in the Department of Basic Science at the University of Tokyo.
Using spectroscopic techniques, scientists have already detected silicon-containing molecules in the gaseous clouds that envelop some stars and in the sparsely populated space between stars. In space, silicon is often found in dust grains containing stable compounds called silicates. However, highly reactive molecules, such as SiCN, have also been detected in the gas phase in the interstellar medium.
Searching for More Reactive Silicon Compounds
Endo and his colleagues wondered if compounds in the same family as SiCN, but with longer carbon chains, also existed in the interstellar medium. But there was big obstacle to answering the question: Researchers had not yet performed any laboratory experiments to determine the spectroscopic signatures of reactive, silicon and nitrogen-terminated carbon chain molecules.
To fill the knowledge gap, Endo and his team created molecules of SiC2N and SiC3N by mixing precursor gases in a supersonic jet and zapping the mixture with electric pulses. The researchers then measured the electromagnetic emissions of the molecules in a Fourier transform microwave spectrometer. To find the peaks in the emission spectrum, the researchers were guided by theoretical calculations.
"Our experiment now makes it possible to search for SiC2N and SiC3N in the interstellar medium," Endo said.
Space Chemistry Insights
Endo and his colleagues plan to use their new results to look for silicon and nitrogen-terminated carbon chain molecules in the gaseous cloud surrounding a giant infrared star called IRC+10216. Scientists had previously detected the single carbon SiCN surrounding this star.
"If [SiC2N and SiC3N] molecules are identified in astronomical objects and their abundances are determined, we will be able to obtain valuable information on the mechanisms for the formations of these molecules," Endo said. "In addition, the information may provide clues to understand formation pathways of other silicon-bearing molecules." The new information could give scientists clues about the chemical composition of the universe and the conditions that birth stars and planets.
More information: The article, "Laboratory detections of SiC2N and SiC3N by Fourier transform microwave spectroscopy" is authored by Hiroya Umeki, Masakazu Nakajima and Yasuki Endo. It will be published in The Journal of Chemical Physics on November 11, 2014. DOI: 10.1063/1.4900740
Journal information: Journal of Chemical Physics
Provided by American Institute of Physics