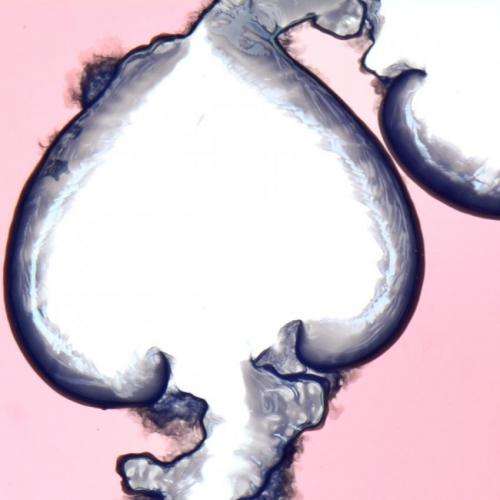
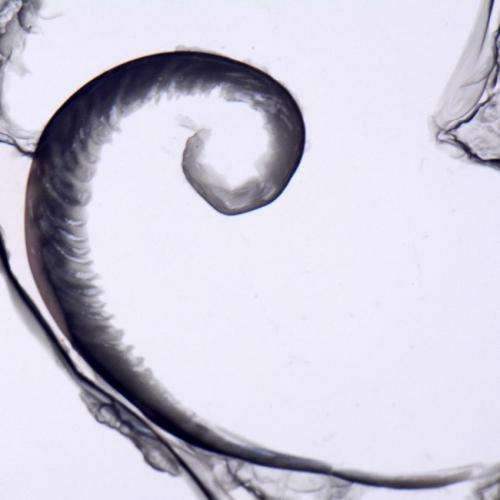
Spiral precipitation pattern in a quasi-2D chemical garden. Credit: Florence Haudin.
(Phys.org) —A team of researchers working at Université libre de Bruxelles in Brussels has devised a means for studying the growth of near 2D chemical gardens in a lab. In their paper published in Proceedings of the National Academy of Sciences, the researchers discuss the difficulty in studying 3D chemical gardens and the advantages of using their method to learn more about the processes involved in their growth.
A chemical garden is a term used to describe the result of growth of plant-looking mineral formations that occur naturally in a variety of settings, chimneys at hydrothermal vents, for example. Scientists have used such formations as a model to create useful products, such as microfluidic devices, fuel cells and catalysts. But because of the complexity of the factors involved in their development (reaction–diffusion processes, buoyancy, osmosis, etc.) little has been learned about what really goes on as the growth occurs. In this new effort, the researchers sought to simplify the process a little bit by constraining the growth to a near 2D space, thus eliminating some of the factors and allowing for closer examination of the growth as it occurs.
The researchers trapped a very thin layer of waterglass (a sodium or potassium silicate solution) between two acrylic plates and then injected cobalt chloride into it, causing a crystalline growth to commence in near 2D. Filming the growth allowed the team to watch as many different shapes (flowers, spirals, terraces, filaments, worms, hairs, lobes, etc.) emerged depending on the concentration of the reagents and how fast the cobalt chloride was injected. They noted that when one of the reagents had a higher concentration, the result was a flower-like growth. If both were highly concentrated, on the other hand, the results tended to look more like long thin filaments.
Confining the garden growth, the researchers note, to near 2D, reduces the spatial freedom and how much influence buoyancy can exert as growth occurs and allows for the use of research tools that are restricted to two-dimensional geometry.
Spiral precipitation pattern in a quasi-2D chemical garden. Credit: Florence Haudin
Besides creating impressive looking forms of natural art, the researchers suggest their technique could be used by other researchers looking to better understand chemical garden growth in real-world applications such as self-assembling nanostructures.
Spiral precipitation pattern in a quasi-2D chemical garden. Credit: Florence Haudin
More information: Spiral precipitation patterns in confined chemical gardens, PNAS, Florence Haudin, DOI: 10.1073/pnas.1409552111
Abstract
Chemical gardens are mineral aggregates that grow in three dimensions with plant-like forms and share properties with self-assembled structures like nanoscale tubes, brinicles, or chimneys at hydrothermal vents. The analysis of their shapes remains a challenge, as their growth is influenced by osmosis, buoyancy, and reaction–diffusion processes. Here we show that chemical gardens grown by injection of one reactant into the other in confined conditions feature a wealth of new patterns including spirals, flowers, and filaments. The confinement decreases the influence of buoyancy, reduces the spatial degrees of freedom, and allows analysis of the patterns by tools classically used to analyze 2D patterns. Injection moreover allows the study in controlled conditions of the effects of variable concentrations on the selected morphology. We illustrate these innovative aspects by characterizing quantitatively, with a simple geometrical model, a new class of self-similar logarithmic spirals observed in a large zone of the parameter space.
Journal information: Proceedings of the National Academy of Sciences
© 2014 Phys.org