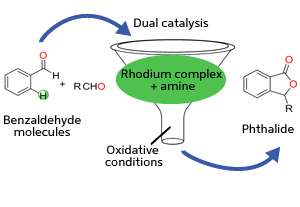
combination of transition metal and organic catalysts can help benzaldehyde molecules to combine into bioactive phthalides in a single step. Credit: A*STAR Institute of Chemical and Engineering Sciences
Plants and herbs containing phthalide molecules—organic compounds that fuse aromatic benzene rings with cyclic, oxygen-bearing hydrocarbons known as lactones—have been used since ancient times as natural medicines to treat diseases such as cardiovascular ailments. Chemists have recently discovered that phthalides can act as valuable 'scaffolds' in the construction of pharmaceuticals with complex structures and more potent capabilities. Unfortunately, phthalides are normally produced through multistep synthetic pathways that can significantly increase manufacturing costs.
Jayasree Seayad and co-workers from the A*STAR Institute of Chemical and Engineering Sciences in Singapore have now developed a novel catalytic process that can generate numerous phthalide compounds in a single step from readily available feedstocks—a 'green' chemistry approach that simultaneously boosts synthetic efficiency and reduces production of environmentally hazardous waste.
One way that chemists are trying to improve the effectiveness of their reactions is with carbon–hydrogen (C–H) activation. Typically, C–H bonds are inert and require harsh conditions to cleave. By contrast, C–H activation reactions can transform these bonds into new functional groups under very mild conditions using catalytically active transition metals such as rhodium complexes.
Seayad and her team anticipated that benzaldehyde—a common benzene derivative bearing a simple carbonyl side chain known as an aldehyde—could serve as ideal reagents for phthalide synthesis because of their structural similarity. To achieve this goal, however, the researchers had to find a way to selectively activate the benzaldehyde C–H bond located beside the aldehyde chain. This is a challenge, notes Seayad, because aldehyde groups are poor at 'directing' the transition metal catalyst to this site.
To overcome this obstacle, the team turned to a combination of rhodium and organic amine catalysts (see image). The amine catalyst first activates benzaldehyde by replacing its oxygen atom with nitrogen, forming a functional group known as an imine. The imine bonds rapidly to the rhodium catalyst and positions it for favorable C–H activation. Then, a second benzaldehyde molecule couples with the activated complex to generate the desired phthalide in high yields. Performing this reaction under oxidative conditions ensures easy regeneration of the rhodium–amine system for numerous catalytic cycles.
The researchers found that this strategy enabled both identical and modified benzaldehydes to couple into phthalides, opening the way for chemists to produce a diverse range of these bioactive molecules with ease. "This discovery will lead to simplified synthesis of phthalide intermediates from readily available aldehydes while avoiding multistep pathways," says Seayad.
More information: Tan, P. W., Juwaini, N. A. B. & Seayad, J. "Rhodium(III)-amine dual catalysis for the oxidative coupling of aldehydes by directed C–H activation: Synthesis of phthalides." Organic Letters 15, 5166–5169 (2013). dx.doi.org/10.1021/ol402145m
Journal information: Organic Letters