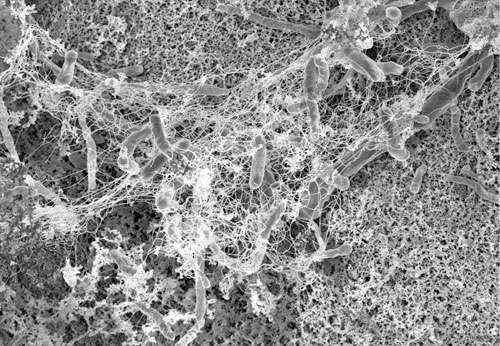
Recent research suggests that some patients develop a potentially deadly blood infection from their implanted cardiac devices because bacterial cells in patients' bodies have gene mutations that allow bacterial cells to stick to the devices. Patients with implants can develop infections because of a biofilm of persistent bacteria on the surfaces of their devices. A biofilm is a community of bacterial cells that lives on the surface of a solid substrate. Biofilms are the most common mode of life for all bacteria, whether they reside in the environment or in the human body. The scientific principles governing the formation of bacterial biofilms on cardiac devices are strongly linked with those of biofilm formation on mineral surfaces. Scientists found that some strains of the bacteria, Staphylococcus aureus, have just a few genetic variants in the proteins on their surfaces that make them more likely to form these biofilms. The team led by scientists at Ohio State University and Duke University Medical Center used atomic force microscopy and powerful computer simulations to determine how Staph bacteria bond to the devices in the process of forming these biofilms.
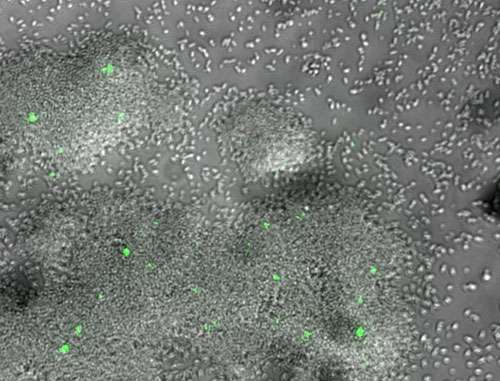
Until now, biofilms—colonies of microbes like bacteria that grow together in a matrix produced by the cells themselves—have been poorly understood. Yet, they can be costly and dangerous. Infections related to hip- and knee-replacement surgery are often related to biofilms. And, biofilms are highly tolerant to antibiotics. Persister cells actually go dormant during treatment and, when the treatment stops, they return and repopulate. Biofilms in oil and water pipelines can rot the metal from inside, destroying infrastructure and potentially introducing contaminants into the water supply.
Coming up with effective ways to control biofilms starts with understanding the complicated communications among biofilm cells, as well as interactions between bacteria and host cells.
With support from the Office of Emerging Frontiers in Research and Innovation within the Engineering Directorate of the National Science Foundation (NSF), one research team working to advance understanding of biofilms is led by Dacheng Ren and colleagues at Syracuse University: Rebecca Bader, Yan-Yeung Luk, Radhakrishna Sureshkumar and Roy Welch.
The team creates synthetic biofilms to better understand their formation and behavior. And, they use supercomputer models to study the molecular structure of biofilms.
"Using molecular dynamics simulations, my coworkers and I in the Sureshkumar research group probe the interaction between the host cell, signaling factors, and the bacterial biofilm matrix based on computer models of the primary host cell and biofilm matrix constituents," explains Stephen DeSalvo, an undergraduate student who works on the modeling. "Using these simulations, lipid bilayer deformation, translocation free energies, and polymer matrix characterization can be analyzed. The real power of these modeling techniques comes with using the physical interactions between system components described via simulations to provide valuable insight into phenomena observed in the biofilm laboratory. Ultimately, both qualitative and quantitative simulation results may aid biofilm and persister cell therapeutic research, as well as future drug delivery breakthroughs."
There are several advantages to having natural microbes break down contaminants in soil and groundwater. It's cost effective and, more importantly, the process involves using native microorganisms that already are present under the earth's surface. Sometimes, however, the microbes need a little boost from the outside to help get them going. Scientists either use "bio-stimulation," which involves giving them nutrients or oxygen, or "bio-augmentation," which involves transferring foreign microbes with new "degradative capabilities" into the existing microbes that will cause them to work. Claudia Gunsch, an assistant professor of civil and environmental engineering at Duke University, is working on providing new genes to existing microbes already under the surface to activate them so they can begin destroying environmental toxins. The process, called bacterial conjugation, involves the transfer of genetic material between bacterial cells by direct cell-to-cell contact or by a bridge-like connection between two cells, known as a pilus. During conjugation, the donor cell provides a conjugative genetic element called a plasmid, which is a specific type of DNA.
The researchers are working on a broad range of approaches to disrupting biofilms—from interrupting the cell communications to manipulating the persister cells to make them more vulnerable to antibiotics. In this multidisciplinary environment, the ideas are infectious!
Provided by National Science Foundation