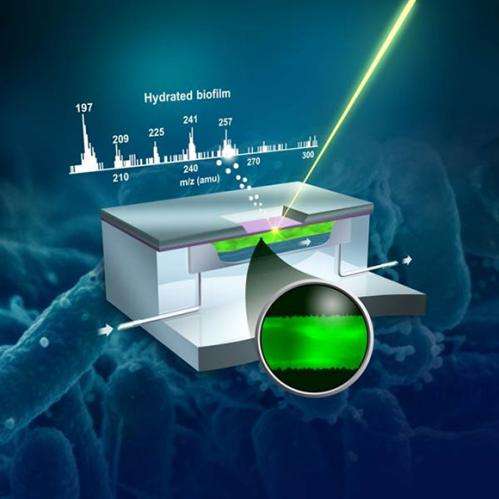
The PNNL microfluidic reactor allows real-time direct observations of hydrated biofilm growth at the molecular level, and is the first to do so. Credit: Royal Society of Chemistry.
(Phys.org) —Catching biofilm chemistry with images has always been a cold or dry affair. Now, a multidisciplinary team at Pacific Northwest National Laboratory is the first to demonstrate imaging of a biofilm's chemical components as they form in hydrated biological samples, rather than from frozen or dried samples. They used a surface technique called time-of-flight secondary ion mass spectrometry, ToF-SIMS, under high vacuum conditions to perform in situ mapping of biofilm chemistry.
The work illustrates that scientists can study complex microbiological processes, such as chemical attachment of microbes to surfaces to form biofilms, using PNNL's vacuum-compatible liquid probe. The study, deemed "the most exciting paper in ToF-SIMS in recent years" by a reviewer, is featured on the cover of Analyst.
Structured microbial communities, or biofilms, are not abstract strangers. Familiar neighbors, biofilms are the plaque on teeth, the residue in shower stalls, and the slippery scum on river rocks, just to name a few. They grow from microbes that join and stick to a surface. Scientists study biofilms to understand how they grow and bond to surfaces, with implications for basic energy, industrial, and biogeochemical research.
The researchers in this study demonstrated a new microfluidic imaging technique that allowed scientists who study biofilms to watch them grow in their native or hydrated form in a vacuum. Previously, chemical imaging of biofilms in a vacuum had to be dried first, which in and of itself changed the anatomy of the sample. The technique described in this research allows the material to be studied in real time, as it grows. And the findings provide a physical foundation to understand this growth at field or even global scales. The work has implications for understanding how natural microbial communities can influence larger biogeochemical processes in subsurface (underground) settings.
"The device allows us to study the biofilm in real-time using surface techniques in a vacuum, and shows the sample in its native form," said Dr. Matthew J. Marshall, PNNL biologist and co-author of the study.
"We are looking at the world from the 'bottom up' for direct observations of molecular level interactions," said PNNL chemist and co-author Dr. Xiao-Ying Yu. "The microfluidic imaging device allows us to observe biofilm growth across different time and length scales."
The team investigated biofilms using a novel liquid vacuum probe capability developed at the Environmental Molecular Science Laboratory (EMSL), a U.S. Department of Energy user facility located at PNNL. They coupled the probe with ToF-SIMS, which revealed that characteristic molecular components could be identified in the microfluidic reactor-grown biofilms. By comparing fragments of biofilm in hydrated samples with dried biofilm samples, their research showed the feasibility of using the innovative microfluidic microreactor for controlled biofilm cultivation and for in situ hydrated, molecular imaging by ToF-SIMS.
Their research provides a new approach to study in situ biofilm physiology at the molecular level and understand complex environmental processes as biofilms interact with surfaces from the molecular to mesoscale dimensions.
This research confirmed the microreactor as a unique approach for correlative imaging of biofilm growth. Ongoing research is looking at purified biofilm components using the same approach to find the differences and similarities between these compounds and intact biofilm.
More information: Hua X, XY Yu, Z Wang, L Yang, B Liu, Z Zhu, AE Tucker, WB Chrisler, EA Hill, S Thevuthasan, Y Lin, S Liu, and MJ Marshall. 2014. "In Situ Molecular Imaging of Hydrated Biofilm in a Microfluidic Reactor by ToF-SIMS." Analyst 139(7):1609-1613. DOI: 10.1039/c3an02262e.
Journal information: Analyst
Provided by Environmental Molecular Sciences Laboratory