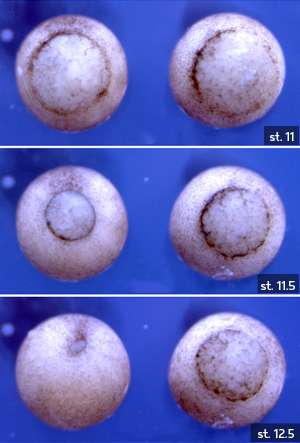
Xenopus embryos without H3.3 (right, shown at stages 11, 11.5 and 12.5) fail to achieve gastrulation. Credit: The Company of Biologists
A specific ratio of DNA packaging proteins ensures normal gene expression during early embryonic development.
Changes in the way that DNA is packaged in the cell can have a dramatic impact on embryonic development. Using a well-established animal model of early development, a team of A*STAR researchers has found that reductions in the level of one particular DNA packaging protein, known as histone H3.3, can lead to abnormal development. By simultaneously depleting another DNA compaction protein called linker histone H1, however, these defects can be corrected.
"Our findings underline the fact that histones are active participants in the process of gene regulation and are not simply inert packaging materials of DNA," says Chin Yan Lim, a developmental biologist at the A*STAR Institute of Medical Biology who led the study.
Lim and her co-workers investigated the role that the histone variant H3.3 plays during embryonic formation in the African clawed frog (Xenopus laevis), which is widely studied in the laboratory to probe questions relating to early development. After blocking the expression of H3.3 using gene-silencing nucleic acid constructs called morpholinos, the researchers observed problems in the formation of the mesoderm, one of the three main tissue types first produced in the embryo. The Xenopus embryos lacking H3.3 then died at a stage of development known as gastrulation (see image).
These developmental defects resulted from a disorganized overall structure of folded DNA, called chromatin, and not from the specific localization of the H3.3 histone variants in the genome. This finding suggests that, in the absence of H3.3, other chromatin components may induce unfavorable DNA arrangements in developing embryos. Indeed, when Lim and her team simultaneously silenced expression of the linker histone H1, they found that the Xenopus embryos developed normally.
As such, the researchers propose that nucleosomal H3 and linker H1 histones have antagonistic effects and that an optimal ratio of H3 to linker histone H1 is needed to allow for proper gene expression and mesodermal development. "How DNA is packaged in the cell is a major way of regulating how cells can react to developmental cues," says Lim. "It is important to identify the other molecular players in this novel pathway to further understand the mechanics of this regulation," she adds.
Beyond furthering scientists' basic understanding of developmental processes, the results could have therapeutic implications. Mutations in histone H3.3 have recently been implicated in some forms of pediatric brain and bone cancers. "Defective chromatin packaging and remodeling is known to contribute to several types of cancers and diseases," Lim says.
More information: Lim, C. Y., Reversade, B., Knowles, B. B. & Solter, D. Optimal histone H3 to linker histone H1 chromatin ratio is vital for mesodermal competence in Xenopus. Development 140, 853–860 (2013).
Journal information: Development