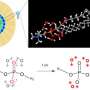
O:H-O bond in water ice. Credit: arXiv:1310.6514 [physics.chem-ph]
(Phys.org) —A team of researchers at Singapore's Nanyang Technological University believes they have solved the mystery of why warm water freezes faster than cooler water. It has to do with the way energy is stored in the hydrogen bonds between water molecules they suggest in their paper which they've uploaded to the preprint server arXiv.
It's long been known that warm water freezes faster than cooler water, (known as the Mpemba effect) going all the way back to Aristotle, but until now, despite a lot of effort by many scientists over thousands of years, no one has been able to offer a reasonable explanation as to why it happens. Now, the team in Singapore appears to have solved the riddle—it's due, they claim, to the small amount of energy stored in stretched hydrogen bonds.
As everyone knows, water molecules have one oxygen atom and two hydrogen atoms—all held together by covalent bonds (the sharing of electrons). What's also known is that with water molecules, hydrogen atoms are also attracted to the oxygen atoms in other nearby water molecules—a force called a hydrogen bond. But, at the same time, the water molecules as a whole are repelled by one another. The team in Singapore has noted that the warmer water gets, the more distance there is between the water molecules due to the repellant force between them. That they say, forces the hydrogen bonds to become stretched out, and stretching out a bond means that there is energy being stored. That energy, the researchers suggest, is released as the water is cooled allowing the molecules to become closer to one another, and (as every chemistry student knows) giving up energy means cooling.
Warm water has more hydrogen bond stretching going on than cool water, thus it stores more energy, and has more to release when exposed to freezing temperatures. That is why, the researchers say, it freezes faster than cool water.
At this point, the claims by the research team are still theory—they or others will still need to find a way to prove what they've suggested is true before the scientific community will deem the mystery of warm water freezing, solved once and for all.
More information: O:H-O Bond Anomalous Relaxation Resolving Mpemba Paradox, arXiv:1310.6514 [physics.chem-ph] arxiv.org/abs/1310.6514
Abstract
We demonstrate that the Mpemba paradox arises intrinsically from the release rate of energy initially stored in the covalent H-O part of the O:H-O bond in water albeit experimental conditions. Generally, heating raises the energy of a substance by lengthening and softening all bonds involved. However, the O:H nonbond in water follows actively the general rule of thermal expansion and drives the H-O covalent bond to relax oppositely in length and energy because of the inter-electron-electron pair coupling [J Phys Chem Lett 4, 2565 (2013); ibid 4, 3238 (2013)]. Heating stores energy into the H-O bond by shortening and stiffening it. Cooling the water as the source in a refrigerator as a drain, the H-O bond releases its energy at a rate that depends exponentially on the initially storage of energy, and therefore, Mpemba effect happens. This effect is formulated in terms of the relaxation time tau to represent all possible processes of energy loss. Consistency between predictions and measurements revealed that the tau drops exponentially intrinsically with the initial temperature of the water being cooled.
Journal information: arXiv
© 2013 Phys.org
![O:H-O bond in water ice. Credit: arXiv:1310.6514 [physics.chem-ph] Researchers claim to have discovered why warm water freezes faster than cooler water](https://scx1.b-cdn.net/csz/news/800a/2013/fbggnhj.jpg)