Small and efficient: Water nanodroplets cool biomolecules ultrafast
Researchers of the Max-Born-Institute at Berlin, Germany, have observed how biomolecules transfer energy into extremely small water droplets in their environment. A water shell consisting of only 3 water molecules around a phospholipid molecule is sufficient for energy transfer within 1 ps.
Biochemical processes occur mainly in an aqueous environment. Particular groups of a biomolecule are embedded in a shell of water molecules, a process called hydration. The water shell stabilizes the biomolecular structure and enables an exchange of energy between the biomolecule and its environment. Examples are the double helix of DNA, the carrier of basic genetic information, in an aqueous medium and the membranes of living cells which consist of phospholipids. The molecular mechanisms, the speed and the efficiency of energy exchange between the biomolecule and the water shell are understood only in part and, thus, a topic of current basic research.
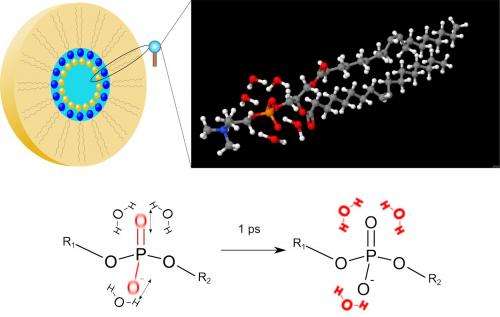
Scientists of the Max-Born-Institute have shown that extremely small water droplets embedding a phospholipid molecule enable efficient energy transfer on a time scale of 1 ps (1 ps = 10-12). René Costard, Christian Greve, Ismael Heisler, and Thomas Elsaesser report in the current issue of Journal of Physical Chemistry Letters (vol.3, page 3646, 2012) that 3 water molecules around the phosphate group of the phospholipid are sufficient for transferring the energy of vibrations from the phospholipid into this minimal water shell. The transferred energy heats the water shell by 10 to 20 centigrades. The thermal energy is stored in tilting motions of water molecules, so called librations, and leads to a weakening of the interaction between the water molecules, the so called hydrogen bonds. The overall molecular structure of the water shell remains practically unchanged. This extremely efficient mechanism of energy disposal allows for the transfer of even larger amounts of energy, protecting the biomolecule against damage by overheating.
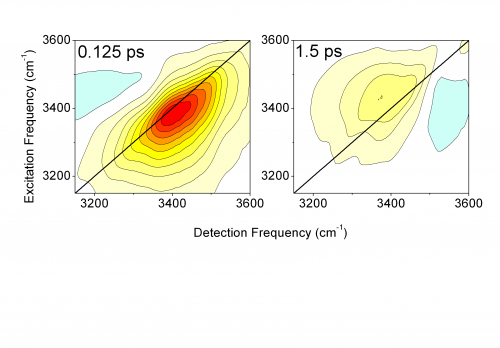
The researchers studied a phospholipid model system consisting of the DOPC molecules shown in Fig. 1. The molecules are arranged in so-called reverse micelles which contain the water molecules hydrating the phosphate groups. In this geometry, the hydration level, i.e., water content, can be changed in a wide range. For studying energy transfer, either phosphate vibrations of the phospholipid or OH stretching vibrations of water are excited by an infrared pulse of a 0.1 ps duration. The vibrations decay within a fraction of a picosecond and the energy released in this decay is transferred into the water shell. The transfer and redistribution of energy is mapped via transient two-dimensional infrared spectra of the OH stretching vibration of water. The weakening of hydrogen bonds in the heated water shell leads to a shift of the OH stretching spectra to higher frequencies. Measuring the change of the two-dimensional spectra as a function of time provides direct insight into the energy transfer dynamics.
Journal information: Journal of Physical Chemistry Letters
Provided by Max-Born-Institute