This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Lanthanide catalysts enable one-step synthesis of complex drug precursors
Hydrocarbazole is a crucial compound in organic chemistry, serving as a building block for various biologically active compounds, including pesticides such as strychnine and anticancer drugs such as vinblastine and minovincine. Consequently, the development of synthesis methods for these compounds is a crucial research topic.
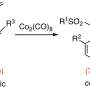
Diels-Alder reaction is one of the most reliable methods for this purpose. For the last decade, a research team from Japan, led by Associate Professor Shinji Harada from the Institute for Advanced Academic Research and the Graduate School of Pharmaceutical Sciences at Chiba University, has conducted extensive research in this field.
Their recent breakthrough employed organic compounds called indole-incorporated siloxydienes and original rare earth catalysts. Despite their progress, there is still room for improvement in terms of the generality of substrates and catalyst reactivity.
In particular, a type of siloxydiene substrate containing a substituent at the second carbon (C2) position of the indole ring is crucial for synthesizing hydrocarbazole compounds with a tetrasubstituted carbon or a carbon atom bonded to four different substituents, at the adjacent position to the nitrogen atom.
A prominent example of such compounds is Kopsinine. It has anticancer and anti-inflammatory properties, garnering significant interest in pharmacological research. However, these siloxydiene substrates have very low reactivity.
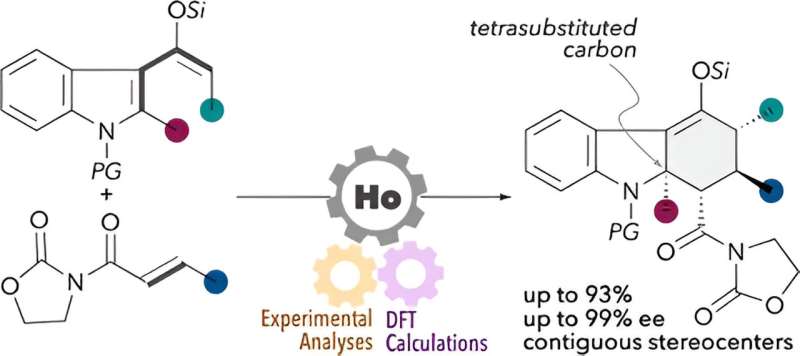
To address this issue, Dr. Harada and his team, including Professor Miki Hasegawa from the Department of Chemistry and Biological Science of the College of Science and Engineering at Aoyama Gakuin University, developed a new technique for synthesizing complex hydrocarbazole compounds with tetrasubstituted carbon.
"Our method uses a new lanthanide-based catalyst and can be used to synthesize complex compounds with high purity. Furthermore, in this method, the lanthanide catalyst can be recycled, thus paving the way for sustainable chemical processes," says Dr. Harada.
Their findings are published in The Journal of Organic Chemistry.
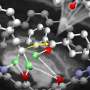
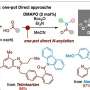
Initially, the researchers attempted to perform the Diels-Alder reaction on a methyl-substituted siloxydiene substrate using a chiral helical ytterbium catalyst that they had previously developed. However, no reaction took place. This was due to the low reactivity of the substrate, which was attributed to the methyl group.
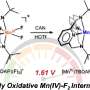
To combat this, they aimed to structurally modify the catalyst to improve its Lewis acidity (the ability to accept electron pairs) and to create space around the central metal. For this, they incorporated the triflimide salt, resulting in a ytterbium triflimide catalyst. They used it to obtain the desired hydrocarbazole compound, albeit with a low yield.
The researchers further modified the catalyst by replacing the central metal, ytterbium, with holmium, a lanthanide metal. The resulting chiral holmium triflimide catalyst significantly improved both the yield to 95% and enantioselectivity, the ability to produce one specific version of the product over its mirror image. In addition, this catalyst could be easily recovered after the reaction.
The researchers synthesized multiple complex hydrocarbazole compounds using this technique, signifying its versatility. Notably, they fabricated a tetracyclic compound with five chiral centers. Furthermore, they studied the reaction mechanisms through both experimental and computational methods.
Highlighting the significance of the study, Dr. Harada remarks, "These findings will contribute to accelerating the development of new drugs. At first glance, this research may seem technical and esoteric; however, its results have the potential to affect every aspect of our lives, including medicine, environment, and food."
By contributing to a sustainable chemical and pharmaceutical industry on a global scale, this research has the potential to improve people's health and quality of life.
More information: Shinji Harada et al, Synthesizing Chiral Hydrocarbazoles with a Tetrasubstituted Carbon Using Holmium-Catalyzed Enantioselective [4 + 2] Cycloaddition: Mechanistic Insights from Luminescence and DFT Studies, The Journal of Organic Chemistry (2024). DOI: 10.1021/acs.joc.4c00837
Provided by Chiba University