This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New study expands the scope of aza-Friedel–Crafts reactions
From life-saving drugs and synthetic polymers to diverse advanced materials, the products containing organic compounds seem endless, thanks in part to regioselectivity, a feature in chemical reactions where a substituent is selectively added to a specific position of an organic compound. This favors the formation of desired products with specific functionalities.
One notable regioselective reaction used for the precise design of organic compounds is the Friedel−Crafts reaction, which enables the addition of substituents to specific positions on aromatic compounds such as benzene and phenol rings.
In the traditional acid-catalyzed aza-Friedel–Crafts reaction of phenols with imines, the substitution reaction typically occurs adjacent to the hydroxy (–OH) group of the phenol ring. This leads to the formation of ortho-products such as α-aminobenzylphenols, which hold significant importance in the production of agrochemicals and pharmaceuticals. However, achieving selective substitution at the para-position further away from –OH is challenging and has only been observed for a few substrates.
In a new study published in the journal ACS Catalysis, Professor Takayoshi Arai from the Graduate School of Science at Chiba University in Japan, along with Masters' students, Mr. Ryoya Tajima and Mr. Takaaki Saito, has made a significant breakthrough by developing a general para-selective aza-Friedel−Crafts type reaction of phenols with imines. Their work has opened up new possibilities for the rapid and safe synthesis of medicines and advanced materials.
"There has been no general way to achieve a para-selective aza-Friedel−Crafts reaction of phenols until now," says Prof. Arai, highlighting the novelty of the present work.
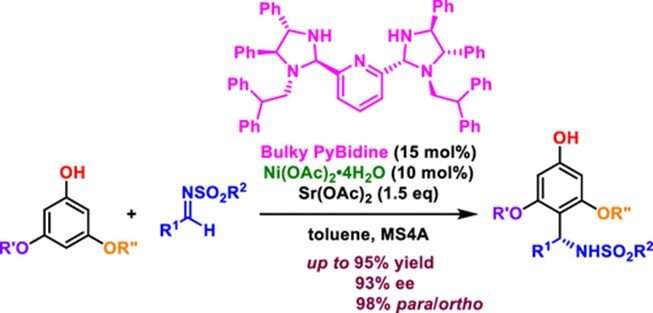
In the traditional aza-Friedel−Crafts reaction, ortho-products are formed in the presence of a Lewis acid catalyst. The challenge faced by the researchers was to steer the reaction towards favoring para-substituted products. To solve this issue, they took inspiration from their earlier experiments, in which they had observed that the bis(imidazolidine) pyridine (PyBidine)-metal complex platform had the potential to carry out the aza-Friedel−Crafts reaction of phenols with imines due to its ability to function both as an acid and a basic catalyst.
In a series of tests, the researchers found in their present work that the PyBidine-Ni(OAc)2 complex showed a preference for para-substituted products. They then replaced the benzyl substituent of PyBidine with a bulky diphenylethyl group to obtain a "bulky PyBidine"-Ni(OAc)2 catalyst, which facilitated highly para-selective aza-Friedel−Crafts reactions with up to 99:1 para/ortho selectivity.
Furthermore, 3,5-dialkoxyphenols with various sulfonylaldimines yielded para-substituted products—with up to 93% enantiomeric excess (chiral purity)—in the presence of a Sr(OAc)2 additive.
By employing DFT calculations, the researchers found that this regioselectivity was due to a cooperative activation of the highest occupied molecular orbital (HOMO) of 3,5-dialkoxyphenols and the lowest unoccupied molecular orbital (LUMO) of sulfonylaldimines.
The reaction began with the formation of a nickel phenoxide, which enhanced the reactivity at the para-position of the phenol group in a process called HOMO activation. The sulfonylaldimine was then attached to this position via hydrogen bonds on the bulky PyBidine-Ni(OAc)2 catalyst through LUMO activation, resulting in the formation of the final product.
This HOMO–LUMO activation was the key to switching the regioselectivity for the design and development of functional molecules for medicines, agrochemicals, and other materials.
In effect, this study is a significant step in the field of regioselective reactions for future applications in various industries. "The rapid and safe synthesis method has improved the synthetic efficiency of an orexin antagonist and is expected to contribute to an environmentally benign and sustainable chemistry," concludes Prof. Arai.
More information: Ryoya Tajima et al, Asymmetric para-Selective aza-Friedel–Crafts Reaction of Phenols Catalyzed by Bulky PyBidine-Ni(OAc)2, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c01961
Journal information: ACS Catalysis
Provided by Chiba University