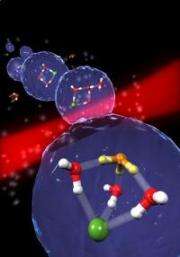
Scientists have discovered the process that causes the formation of tiny acid droplets (whose structure is shown) in the stratosphere, which leads to ozone depletion. Image: Bochum University, Germany.
(PhysOrg.com) -- In its atomic form, chlorine can destroy vast quantities of ozone. But exactly how chlorine is created in the ultracold conditions of the stratosphere has puzzled scientists. Now, a team of researchers from Italy and Germany has found a mechanism for how chlorine can easily form in these unfavorable conditions, which is based on water's ability to bond with a strong acid.
In their study, which is published in a recent issue of Science, Marco Masia of the SLACS Laboratory of the Italian INFM-CNR, along with Martina Havenith and Dominik Marx from the University of Rurh Bochum in Germany, and their coauthors have observed the fundamental chemical process of acid dissociation in ultracold conditions. Dissociation is the process by which molecules split into their smaller constituents. In this case, the scientists investigated how hydrogen chloride (HCl) can be dissociated into a negative chloride ion and positive hydrogen ion (proton) by adding just four molecules of water (H2O) to form a tiny droplet of hydrochloric acid.
This observation is surprising, since scientists have previously thought that a lot of energy is required for hydrogen chloride to dissociate in the freezing conditions of the stratosphere. At cold temperatures, molecules wouldn't have enough thermal energy to undergo such a chemical reaction. But the new observation shows that a new reaction mechanism can also enable the formation of molecules - not only in the stratosphere, but also in interstellar space and on the surface of nanocrystals of ice.
In their experiments, the scientists used helium droplets as a "nanoscale laboratories," inside of which they captured HCl and H2O molecules. As the helium droplets absorbed these molecules one by one, the molecules could bind together to form molecular clusters, allowing the researchers to control the size of the clusters. The scientists cooled the helium droplets to -272° C, and then used laser-based infrared spectroscopy to discover how the chemical process begins.
As the researchers explain, the key to the dissociation is stepwise aggregation, or adding one water molecule at a time. When the molecular cluster consists of three water molecules and one hydrogen chloride molecule, the water molecules orient themselves to accept protons from the hydrogen in the hydrogen chloride, but the cluster is still undissociated.
Upon adding a fourth water molecule, the hydrogen chloride spontaneously becomes dissociated. That is, the hydrogen (proton) dissociates from chlorine and binds to a water molecule to form hydronium, leaving the free negative chloride ion.
"These chemical components join themselves in a peculiar structure: the hydronium on one side of a grid, the chloride on the other, and the three water molecules joined as a barrier between the two," the scientists said in a press release. They explained that this structure is the only stable configuration, and also marks the smallest acid droplet possible.
The team calculated that, due to the step-by-step aggregation that takes place at low temperatures, this dissociation process can occur without the need for large amounts of energy.
"Molecules at that cold temperature follow a stepwise mechanism caused by their mutual attraction: as they move to come close one to the other, they eventually acquire enough energy to become chemically active," the scientists explained. "This aggregation, then, makes chemical reactions possible at ultralow temperatures."
More information: "Aggregation-Induced Dissociation of HCl(H2O)4 Below 1 K: The Smallest Droplet of Acid." Science, Vol. 324, Issue 5934, June 19, 2009. DOI: 10.1126/science.1171753
© 2009 PhysOrg.com