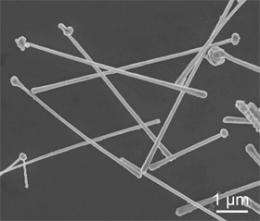
Tiny copper wires can be built in bulk and then "printed" on a surface to conduct current, transparently. Benjamin Wiley, Duke Chemistry
(PhysOrg.com) -- A team of Duke University chemists has perfected a simple way to make tiny copper nanowires in quantity. The cheap conductors are small enough to be transparent, making them ideal for thin-film solar cells, flat-screen TVs and computers, and flexible displays.
"Imagine a foldable iPad," said Benjamin Wiley, an assistant professor of chemistry at Duke. His team reports its findings online this week in Advanced Materials.
Nanowires made of copper perform better than carbon nanotubes, and are much cheaper than silver nanowires, Wiley said.
The latest flat-panel TVs and computer screens produce images by an array of electronic pixels connected by a transparent conductive layer made from indium tin oxide (ITO). ITO is also used as a transparent electrode in thin-film solar cells.
But ITO has drawbacks: it is brittle, making it unsuitable for flexible screens; its production process is inefficient; and it is expensive and becoming more so because of increasing demand.
"If we are going to have these ubiquitous electronics and solar cells," Wiley said, "we need to use materials that are abundant in the earth's crust and don't take much energy to extract." He points out that there are very few materials that are known to be both transparent and conductive, which is why ITO is still being used despite its drawbacks.
However, Wiley's new work shows that copper, which is a thousand times more abundant than indium, can be used to make a film of nanowires that is both transparent and conductive.
Silver nanowires also perform well as a transparent conductor, and Wiley contributed to a patent on the production of them as a graduate student. But silver, like indium, is rare and expensive. Other researchers have been trying to improve the performance of carbon nanotubes as a transparent conductor, but without much luck.
"The fact that copper nanowires are cheaper and work better makes them a very promising material to solve this problem," Wiley said.
Wiley and his students, PhD candidate Aaron Rathmell and undergraduate Stephen Bergin, grew the copper nanowires in a water-based solution. "By adding different chemicals to the solution, you can control the assembly of atoms into different nanostructures," Wiley said. In this case, when the copper crystallizes, it first forms tiny "seeds," and then a single nanowire sprouts from each seed. It's a mechanism of crystal growth that has never been observed before.
Because the process is water-based, and because copper nanowires are flexible, Wiley thinks the nanowires could be coated from solution in a roll-to-roll process, like newspaper printing, which would be much more efficient than the ITO production process.
Other researchers have produced copper nanowires before, but on a much smaller scale.
Wiley's lab is also the first to demonstrate that copper nanowires perform well as a transparent conductor. He said the process will need to be scaled up for commercial use, and he's got a couple of other problems to solve as well: preventing the nanowires from clumping, which reduces transparency, and preventing the copper from oxidizing, which decreases conductivity. Once the clumping problem has been worked out, Wiley believes the conductivity of the copper nanowires will match that of silver nanowires and ITO.
Wiley, who has applied for a patent for his process, expects to see copper nanowires in commercial use in the not-too-distant future. He notes that there is already investment financing available for the development of transparent conductors based on silver nanowires.
"We think that using a material that is a hundred times cheaper will be even more attractive to venture capitalists, electronic companies and solar companies who all need these transparent electrodes," he said.
Provided by Duke University