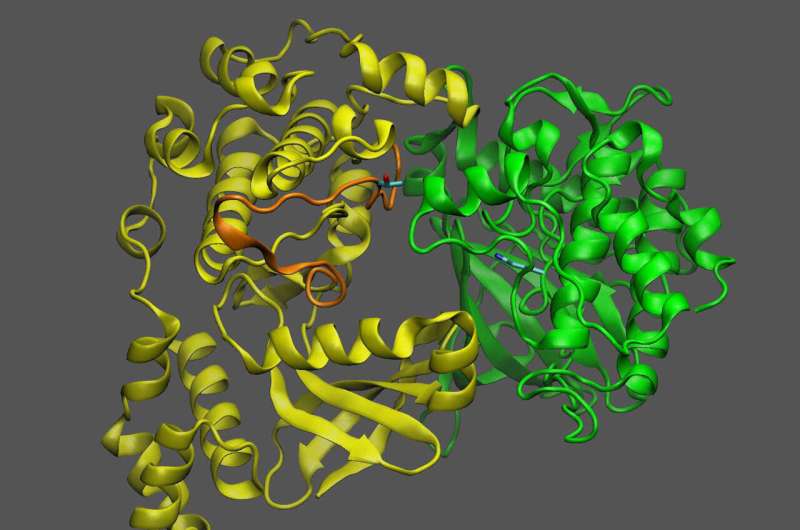
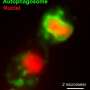
This image shows a plant protein known as KIN10 (yellow) that acts as a sensor and a switch to turn oil production off or on depending on whether it interacts with another protein (green). Credit: Brookhaven National Laboratory
Proteins are molecular machines, with flexible pieces and moving parts. Understanding how these parts move helps scientists unravel the function a protein plays in living things—and potentially how to change its effects. Biochemists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory and colleagues at DOE's Pacific Northwest National Laboratory (PNNL) have published a new example of how one such molecular machine works.
Their paper in the journal Science Advances describes how the moving parts of a particular plant protein control whether plants can grow and make energy-intensive products such as oil—or instead put in place a series of steps to conserve precious resources. The study focuses specifically on how the molecular machinery is regulated by a molecule that rises and falls with the level of sugar—plants' main energy source.
"This paper reveals the detailed mechanism that tells plant cells, 'we have lots of sugar,' and then how that signaling affects the biochemical pathways that trigger processes like plant growth and oil production," said Brookhaven Lab biochemist Jantana Blanford, the study's lead author.
The study builds on earlier work by the Brookhaven team that uncovered molecular links between sugar levels and oil production in plants. One potential goal of this research is to identify specific proteins—and parts of proteins—scientists can engineer to make plants that produce more oil for use as biofuels or other oil-based products.
"Identifying exactly how these molecules and proteins interact, as this new study does, brings us closer to identifying how we might engineer these proteins to increase plant oil production," said John Shanklin, chair of Brookhaven Lab's Biology Department and leader of the research team.
Unraveling molecular interactions
The team used a combination of laboratory experiments and computational modeling to zero in on how the molecule that serves as a sugar proxy binds to a "sensor kinase" known as KIN10.
KIN10 is the protein that contains the moving parts that determine which biochemical pathways are on or off. The scientists already knew that KIN10 acts as both a sugar sensor and a switch: When sugar levels are low, KIN10 interacts with another protein to set off a cascade of reactions that ultimately shut down oil production and break down energy-rich molecules like oil and starch to make energy that powers the cell.
But when sugar levels are high, KIN10's shut-down function is shut off—meaning plants can grow and make lots of oil and other products with the abundant energy.
But how does the sugar proxy binding to KIN10 flip the switch?
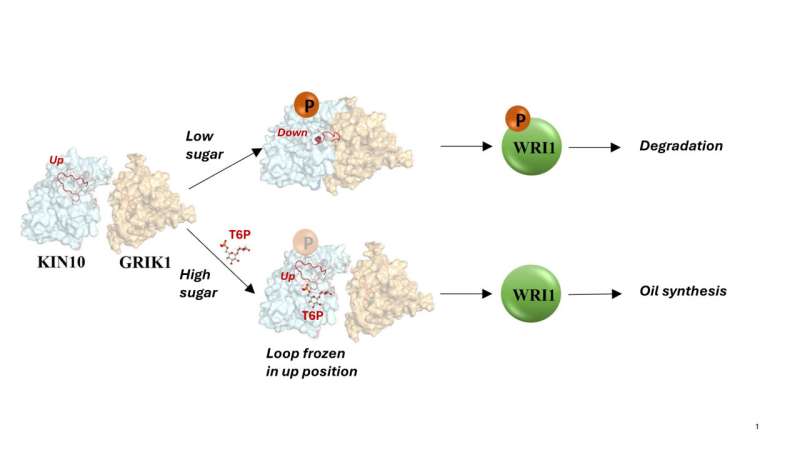
This diagram shows the two pathways KIN10 and an adjacent protein, GRIK1, follow in the low- and high-sugar conditions. Low sugar allows the addition of a phosphate (P) to KIN10, which triggers a phosphorylation cascade that leads to the breakdown of enzymes involved in oil synthesis. This includes degradation of WRI1, the master-switch for oil synthesis. When sugar is abundant, however, a sugar-proxy molecule (T6P) binds to the KIN10 loop to block its interaction with GRIK1. That keeps the oil synthesis pathway open. Credit: Brookhaven National Laboratory
To find out, Blanford started with the adage of "opposites attract." She identified three positively charged parts of KIN10 that might be attracted to abundant negative charges on the sugar proxy molecule. A laboratory-based process of elimination that involved making variations of KIN10 with modifications to these sites identified the one true binding site.
Then the Brookhaven team turned to computational colleagues at PNNL.
Marcel Baer and Simone Raugei at PNNL examined at the atomic level how the sugar proxy and KIN10 fit together.
"By using multiscale modeling we observed that the protein can exist in multiple conformations but only one of them can effectively bind the sugar proxy," Baer said.
The PNNL simulations identified key amino acids within the protein that control the binding of the sugar. These computational insights were then confirmed experimentally.
The combined body of experimental and computational information helped the scientists understand how interaction with the sugar proxy directly affects the downstream action of KIN10.
Flipping the switch
"Additional analyses showed that the entire KIN10 molecule is rigid except for one long flexible loop," Shanklin said. The models also showed that the loop's flexibility is what allows KIN10 to interact with an activator protein to trigger the cascade of reactions that ultimately shut down oil production and plant growth.
When sugar levels are low, and little sugar proxy molecule is present, the loop remains flexible, and the shutdown mechanism can operate to reduce plant growth and oil production. That makes sense to conserve precious resources, Shanklin said.
This animation shows how a flexible loop (orange) on a plant protein known as KIN10 (yellow) allows it to interact with another protein (green) — but only when sugar levels are low. The interaction of the two proteins triggers a cascade of reactions that break down other proteins involved in oil synthesis so the plant can conserve its resources. When sugar levels are high, meaning the plant has abundant resources, a sugar-proxy molecule blocks the loop's swinging motion. That prevents the protein interaction, which keeps the oil-production pathway open. Credit: Brookhaven National Laboratory
But when sugar levels are high, the sugar proxy binds tightly to KIN10.
"The calculations show how this small molecule blocks the loop from swinging around and prevents it from triggering the shutdown cascade," Blanford said.
Again, this makes sense since abundant sugar is available for plants to make oil.
Now that the scientists have this detailed information, how might they put it to use?
"We could potentially use our new knowledge to design KIN10 with altered binding strength for the sugar proxy to change the set point at which plants make things like oil and break things down," Shanklin said.
More information: Jantana Blanford et al, Molecular mechanism of trehalose 6-phosphate inhibition of the plant metabolic sensor kinase SnRK1, Science Advances (2024). DOI: 10.1126/sciadv.adn0895. www.science.org/doi/10.1126/sciadv.adn0895
Journal information: Science Advances
Provided by Brookhaven National Laboratory