(Phys.org) —A Purdue University-led team has revealed the proton transfer pathway responsible for a majority of energy storage in photosynthesis. Through photosynthesis, plants, algae and bacteria convert sunlight, carbon dioxide and water into chemical energy stored in the membrane of special cells, a process similar to charging a battery, said William A. Cramer, the Henry Koffler Distinguished Professor of Biological Sciences and research team leader.
"The key to photosynthesis is the movement of electrical charge from a positive to a negative pole, just as in a battery," Cramer said. "In this case, the electrical charge is in the form of electrons and protons passed along by amino acids and water molecules in a 'bucket brigade' through the cellular membrane. We identified and found the structure and orientation of the individual bucket carriers."
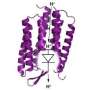
His team looked at the pathway through the cytochrome complex, a group of eight proteins responsible for transporting two-thirds of the protons that energize the plant cell "battery." The proteins are made up of different sequences of amino acids, including those that participate in the "bucket brigade" of proton transfer.
Cramer credits S. Saif Hasan, a graduate student in his research group who will receive his doctoral degree in May, with leading the project.
The team used X-ray crystallography to describe the molecular structure of the cytochrome complex isolated from cyanobacteria, the most primitive photosynthetic organism.
A paper detailing the National Institutes of Health-funded work was published in the Proceedings of the National Academy of Sciences.
Although a mechanism for energy storage involving transfer of protons across biological membranes was the subject of the 1978 Nobel Prize in chemistry and advances had been made in its understanding, the amino acids involved and how they are connected for proton transfer in the photosynthetic protein complex was unknown, Cramer said.
In addition to Cramer and Hasan, team members include E. Yamashita of the Institute for Protein Chemistry in Osaka, Japan, and D. Baniulis of the Lithuanian Research Institute for Agriculture and Forestry.
Understanding details of the process of photosynthesis aids work toward the development of artificial photosynthesis, which could allow for the conversion of solar energy into alternative environmentally friendly sources of biofuels.
The findings also contribute to the understanding of membrane proteins, which regulate all traffic into and out of the cell and are important for drug delivery and structural biology.
Membrane proteins are fat-soluble, which makes them especially difficult to isolate and crystallize for examination, Cramer said.
"Membrane proteins dissolve only in fat, not water, and if you pull them out of the cell membrane they tend to congeal like grease on a frying pan dipped in cold water," he said. "Scientists have only been able to determine the structure of relatively few of this group of proteins, and we have much more to learn."
More information: Quinone-Dependent Proton Transfer Pathways in the Photosynthetic Cytochrome b6f Complex, S. Saif Hasan, Eiki Yamashita, Danas Baniulis, and William A. Cramer, Proceedings of the National Academy of Sciences, 2013.
ABSTRACT
As much as two-thirds of the proton gradient used for transmembrane free energy storage in oxygenic photosynthesis is generated by the cytochrome b6f complex. The proton uptake pathway from the electrochemically negative (n) aqueous phase to the n-side quinone binding site of the complex, and a probable route for proton exit to the positive phase resulting from quinol oxidation, are defined in a 2.70-Å crystal structure and in structures with quinone analog inhibitors at 3.07 Å (tridecyl-stigmatellin) and 3.25 Å (2-nonyl-4-hydroxyquinoline N-oxide) resolution. The simplest n-side proton pathway extends from the aqueous phase via Asp20 and Arg207 (cytochrome b6 subunit) to quinone bound axially to heme cn. On the positive side, the heme-proximal Glu78 (subunit IV), which accepts protons from pastosemiquinone, defines a route for H+ transfer to the aqueous phase. These pathways provide a structure-based description of the quinone-mediated proton transfer responsible for generation of the transmembrane electrochemical potential gradient in oxygenic photosynthesis
Journal information: Proceedings of the National Academy of Sciences
Provided by Purdue University