The average price for all types of gasoline is holding steady around $2.95 per gallon nationwide, but the pain at the pump might be short-lived as research from the University of Houston may eliminate one of the biggest hurdles to the wide-scale production of fuel cell-powered vehicles.
Peter Strasser, an assistant professor of chemical and biomolecular engineering, led the research team in discovering a method to make a fuel cell more efficient and less expensive. The initiative is one of four ongoing fuel cell projects in development at the Cullen College of Engineering at UH.
A fuel cell converts chemically stored energy directly into electricity and is already two to three times more efficient in converting fuel to power than the internal combustion engine usually found in automobiles.
“A fuel cell is a power generation device that converts energy into electricity with very high efficiencies without combustion, flame, noise or vibration,” Strasser said. “If a fuel cell is run on hydrogen and air, as planned for automotive fuel cells, hydrogen and oxygen molecules combine to provide electricity with water as the only byproduct.”
The key to making a fuel cell work is a catalyst, which facilitates the reaction of hydrogen and oxygen. The most common, but expensive, catalyst is platinum. Currently, the amount of platinum catalyst required per kilowatt to power a fuel cell engine is about 0.5 to 0.8 grams, or .018 to .028 ounces. At a cost of about $1,500 per ounce, the platinum catalyst alone would cost between $2,300 to $3,700 to operate a small, 100-kilowatt two- or four-door vehicle – a significant cost given that an entire 100-kilowatt gasoline combustion engine costs about $3,000. To make the transition to fuel cell-powered vehicles possible, the automobile industry wants something better and cheaper.
“The automobile companies have been asking for a platinum-based catalyst that is four times more efficient, and, therefore, four times cheaper, than what is currently available,” Strasser said. “That’s the magic number.”
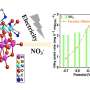
Strasser and his team, which includes Ratndeep Srivastava, a graduate student, Prasanna Mani, a postdoctoral researcher, and Nathan Hahn, a 2007 UH graduate, have met and, seemingly, exceeded this “magic number.” The team created a catalyst that uses less platinum, making it at least four times – and up to six times – more efficient and cheaper than existing catalysts at comparable power levels.
“We have found a low platinum alloy that we pre-treat in a special way to make it very active for the reaction of oxygen to water on the surface of our catalyst,” Strasser said. “A more active catalyst means that we get more electricity, or energy, for the amount of platinum used and the time it’s used for. With a material four to six times more efficient, the cost of the catalyst has reached an important target set by industrial fuel cell developers and the U.S. Department of Energy.”
Although more testing of how the durability of this new catalyst compares to pure platinum is necessary, the preliminary results look promising.
“The initial results show that durability is improved over pure platinum, but only longer-term testing can tell,” Strasser said.
Long-term results may take some time, but industry expert Hubert Gasteiger, a leading scientist in fuel research with Aeta S.p.A. in Italy, is already excited.
“The automotive cost targets, which were developed several years ago, require that the activity of the available platinum catalysts would need to be increased by a factor of four to six,” Gasteiger said. “The novel catalyst concept developed by Professor Strasser’s group has been demonstrated to provide an enhancement factor of greater than four, and, thereby, are very promising materials to achieve the platinum metals cost targets of typical hydrogen-oxygen automotive fuel cells. This is a very exciting and new development, even though more work is required to assure that the durability of these novel catalysts is equally superior to the current carbon-supported platinum catalysts.”
Source: University of Houston