This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Three novel inorganic clusters accelerate chemical reactions to create carbon–carbon bonds
Perfume, rubbing alcohol, a cholesterol medication and even biological processes all depend on a chemical process called the aldol reaction. The reaction primarily combines compounds to form carbon–carbon bonds, which are incredibly strong and provide a molecule with stability.
Catalyst clusters made of aluminum and oxygen typically help accelerate this reaction, but clusters that also include rare earth elements could offer more desirable and synergistic properties, according to a team of researchers based in China.
The team developed three such clusters, each of which produced an actual yield of at least 74% and up to 86% of the theoretical potential final products—which are considered good in practical settings, such as a laboratory. They published their results in Polyoxometalates.
"In organic chemistry, the aldol reaction is one of the most important methods for the formation of carbon-carbon bonds," said corresponding author Wei-Hui Fang, research professor, State Key Laboratory of Structural Chemistry at the Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences. "So far, many catalysts have been used in aldol reactions, but clusters have less used in this regard."
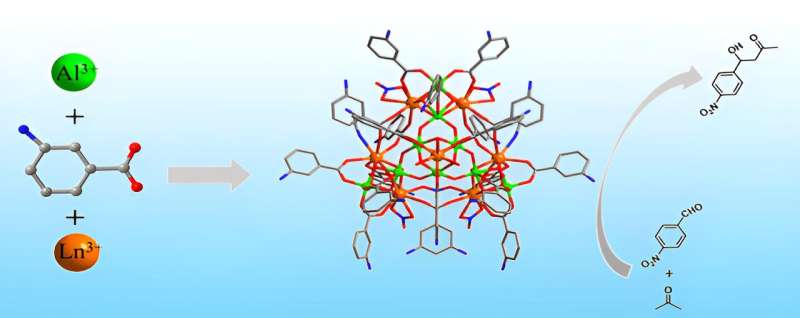
Clusters comprise bound atoms and are bigger than a molecule but smaller than a bulk solid material. Conventional catalytic clusters, known as homometallic complexes, are made with aluminum and oxygen or rare earth elements. However, according to Fang, heterometallic complexes that combine the two are far rarer—despite properties that would allow the two components to work better together.
"Heterometallic compounds can lead to emergent synergistic properties but remain relatively unexplored due to complicated reaction systems containing more than three components including two metals plus linkers," Fang said.
Fang and her team previously developed a method to produce aluminum molecular rings loaded with single lanthanide ions, which are a class of rare earth elements known as light metals. They found that by increasing the amount of aluminum and the lanthanide ions, they could produce a pure cluster compound with crystalline structure. By changing the amount and kind of lanthanide ions—cerium, praseodymium or neodymium—they produced the three heterometallic clusters.
"We employed ligand-controlled partial hydrolysis to produce these hat-shape clusters," Fang said. Such a process involves using water to break molecules into smaller components that can rearrange into different complexes. Ligands, or ions bonded to an atom, can help control the process by preventing certain dissociations. "Its unique vertex-to-edge sharing arrangement has not been reported in either rare earth or aluminum oxo clusters."
The sharing arrangement refers to how the molecules bond together, with edges and vertices pairing in such a way that the clusters look like hats. The researchers used various imaging and chemical analysis techniques to characterize the clusters. They then tested how well each one accelerated an aldol reaction with acetone. At 60°C and after 48 hours, the cluster with cerium produced an 86% yield, which Fang called an "excellent" result. The cluster with praseodymium had an 84% yield, and the cluster with neodymium had a 74% yield.
"It can be seen that heterometallic combination of aluminum and the rare earth system brings a completely different structure type from the two pristine systems," Fang said. "We anticipate that the ligand-controlled partial hydrolysis will continue to be effective in heterometallic synthesis."
More information: Xiao-Yu Liu et al, Tetrameric cubane Al 9Ln 7 (Ln = Ce, Pr, Nd) clusters as aldol addition catalysts, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140045
Provided by Tsinghua University Press