This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
New family of wheel-like metallic clusters exhibit unique properties
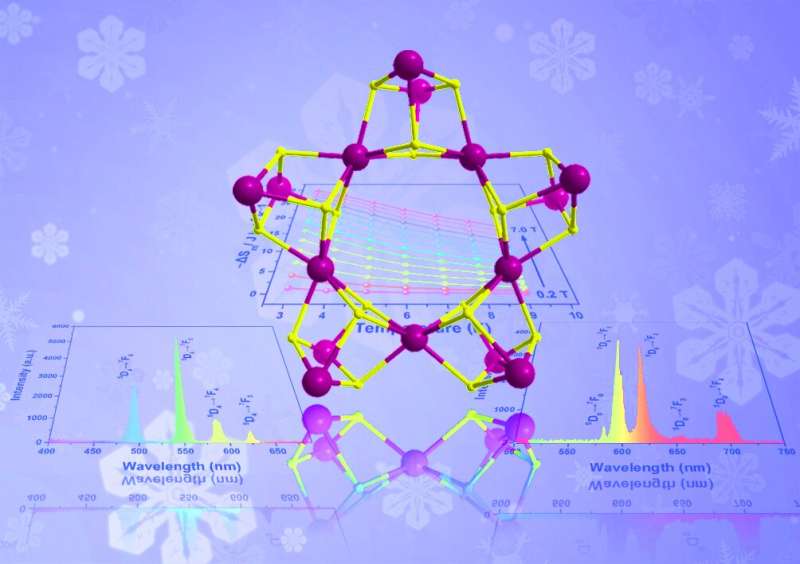
While the wheel does not need to be reinvented, there are benefits to the development of new nano-wheels, according to a multi-institute research team based in China. The group fabricated a novel family of metallic compounds, each of which exhibit unique properties desirable for next-generation technologies, such as advanced sensors.
Their findings were made available in Polyoxometalates.
"Polymetallic complexes are of great interest not only for their appealing molecular structure but also for their versatile applications in various fields," said co-corresponding author Yan-Zhen Zheng, professor in the Frontier Institute of Science and Technology (FIST) at Xi'an Jiaotong University.
Polymetallic complexes, which comprise multiple atoms of various metals or a combination of metals and other elements, have the potential to imbue materials with specific properties if the molecules can be synthesized, Zheng said. Such properties include the ability to fluoresce, or glow, and magnetic quirks that allow drastic temperature changes and control.
Zheng and his team focused on creating polymetallic complexes made with lanthanide elements, a group of 15 metallic materials also known as rare earth elements. They specifically used europium, terbium and gadolinium.
"Among all polymetallic complexes, lanthanide-based compounds have drawn unprecedented attention due to their interesting magnetic and luminescence behaviors," Zheng said. "Several such compounds have been successfully isolated, but direct synthesis has been a challenge."
The components of the complexes require are geometrically diverse, requiring significant coordination, according to Zheng.
"Previous findings revealed that controlling the hydrolysis—breaking down a compound with water—of lanthanide metal ions in the presence of appropriate organic ligands would be a powerful strategy to obtain desired species," Zheng said. A ligand is a molecule that bonds to a metal atom. Its addition to the complex can stabilize the structure.
The researchers used hydrolysis to breakdown lanthanides in a bath containing a ligand called tricine. Tricine contains multiple arms of oxygen and hydrogen, meaning it can accommodate a large range of metals and help stabilize the resulting clusters.
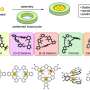
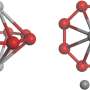
"Through the simple hydrolysis reaction, we synthesized three lanthanide nano-clusters, and used X-ray diffraction analyses to reveal their stable, wheel-like structure," Zheng said. "Owing to the presence of different lanthanide metal ions in these analogues, each compound shows distinctive properties."
The europium-based cluster fluoresced red emissions, while the terbium-based cluster fluoresced green emissions. The gadolinium-based cluster exhibited potential applications in magnetic cooling. According to Zheng, the research group is continuing to investigate the synthesis and application of these clusters.
More information: Peng-Fei Sun et al, Tricine-supported polyoxo(alkoxo)lanthanide cluster {Ln 15} (Ln = Eu, Gd, Tb) with magnetic refrigerant and fluorescent properties, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140026
Provided by Tsinghua University Press