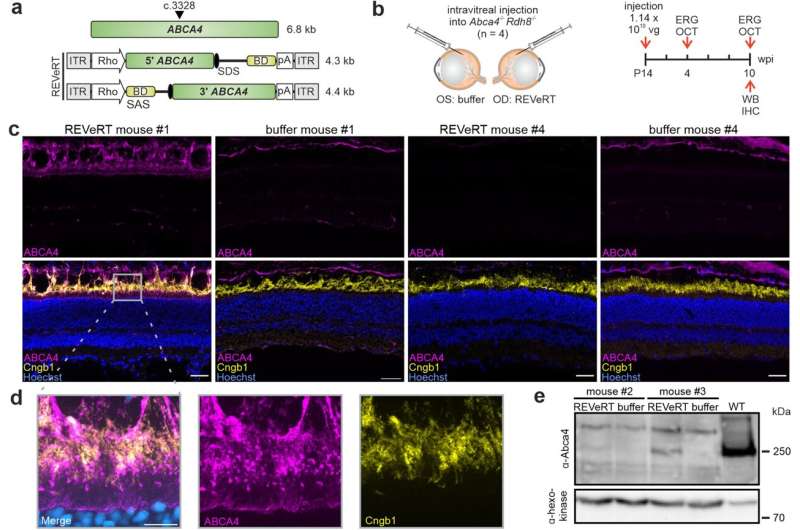
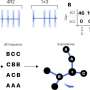
ABCA4 reconstitution and full-length protein expression in a Stargardt mouse model using REVeRT. a Upper panel, ABCA4 size and the split site (arrowhead). Lower panel, Dual REVeRT AAV.GL vectors expressing split ABCA4. b Experimental design for (c–e) and Fig. S7. c Representative immunostainings on retinal sections of mouse #1 (left) and mouse (#4) showing a strong or weak improvement in OCT and ERG measurements, respectively. Images of the right eye injected with dual REVeRT AAVs and images of the contralateral control eye injected with buffer solution are shown. Scale bar: 30 µm. Cyclic nucleotide-gated channel (Cngb1) antibody was used as a rod outer segment marker to evaluate ABCA4 localization. Some unspecific ABCA4 staining was detected across all sections above the outer segments. d Higher magnification of the area marked by a gray rectangle in (c). Scale bar: 10 µm. e Western blot from retinas of mouse #2 and mouse #3. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-42386-0
One problem in gene therapy is that not all genes transfer equally well into the target cells. UZH researchers have now developed a flexible method to transfer large genes efficiently and without significant side effects. The approach has strong potential for therapeutic use.
Gene therapy currently represents the most promising approach for the treatment of hereditary diseases. Yet despite significant breakthroughs in recent years, there are still a number of hurdles that hinder the wider application of gene therapies. These include the efficient delivery of genetic material into target cells with minimal side effects using adeno-associated viral vectors (AAVs).
The AAV carrier substances have an advantageous safety profile and high gene transfer efficiency, meaning they are often used in gene therapies and in gene editing with CRISPR/Cas. But AAVs have limited DNA uptake capacity and cannot reliably transport larger genes.
Various methods have been developed to circumvent these drawbacks. Such methods rely on splitting the coding DNA into two fragments that contain the ability to be rejoined in the target tissue. The disadvantage of these strategies, however, is that they are not very efficient and offer less flexibility for experimental design, and they can cause potential side effects.
The team of Elvir Becirovic, professor of experimental and translational ophthalmology at the University of Zurich, has now developed a novel approach to circumvent these drawbacks. This new method, called REVeRT (reconstitution via mRNA trans-splicing), also uses the principle of dual AAV vectors. However, unlike previous technologies, it relies on the assembly of split gene fragments at the transcript level.
"The advantages of this method are increased efficiency and fewer side effects," explains Becirovic. "It is also more flexible than previous methods, as the large genes can be divided into two fragments at various points." His team has also developed the method for ophthalmologic applications in cell cultures and successfully evaluated it in animal models under various conditions, for example to treat hereditary macular degeneration with gene therapy.
The study is published in the journal Nature Communications.
REVeRT is also suitable for use in gene therapies for other genetic or acquired diseases—such as various common blood disorders or diseases associated with aging. The new method can also find application in gene therapy studies using CRISPR/Cas genome editing. For such modules to be of therapeutic use, the coding DNA needs to be transferred into the target cells as efficiently as possible with the help of carriers such as AAVs. "With CRISPR/Cas further applications are possible, opening up new treatment options," says Becirovic.
More information: Lisa Maria Riedmayr et al, mRNA trans-splicing dual AAV vectors for (epi)genome editing and gene therapy, Nature Communications (2023). DOI: 10.1038/s41467-023-42386-0
Journal information: Nature Communications
Provided by University of Zurich