This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reprogram gene therapy viral vectors to bind specific protein targets
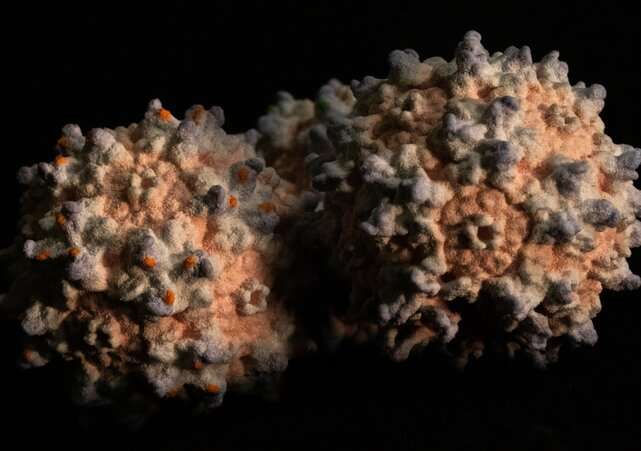
Scientists have engineered adeno-associated viruses (AAVs) to package and deliver gene therapies to cells in the body. But the field has struggled to develop AAVs that can efficiently target different cell types and organs such as the brain, driving scientists to look for better ways of developing new viral vectors.
A team of researchers at the Broad Institute of MIT and Harvard has built a more focused and efficient method of engineering AAVs. Previous methods introduce millions of AAV capsids—the outer shells of the virus that bind to target proteins—into animals and rely on iterative rounds of screening to find AAVs that reach specific cells.
The new approach, developed by the lab of Ben Deverman, an institute scientist and senior director of vector engineering at Broad, instead looks for AAVs that bind to known proteins on the surface of target cells or organs.
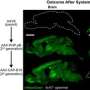
Using their method, the team found AAVs that bind to two proteins on the surface of cells that line blood vessels in the brain, allowing them to cross the blood-brain barrier in mice. The researchers' approach, published in PLOS Biology, could be used to engineer AAVs that target proteins on human cells, potentially accelerating the development of gene therapies.
"This is a really nice demonstration that when we engineer AAVs by designing them to interact with certain proteins, we are better able to predict what their activity will be when they're delivered in vivo," Deverman said. Qin Huang, a senior research scientist in Deverman's group, is the first author on the study.
Protein binding
Existing approaches to AAV engineering are not only labor-intensive but also don't reveal how specific AAVs are able to reach specific cells, often resulting in AAVs that only work in certain species.
"In vivo selection is quite straightforward, but mouse data doesn't necessarily lead to something that will work in humans, which is a problem if you want to use it in gene therapy," Huang said.
In 2019, Deverman's group identified the protein that serves as a receptor for AAV-PHP.B, a capsid that is efficiently transported across the blood-brain barrier. That made them wonder whether they could develop a screening approach that would find more AAVs that interacted with proteins they knew were expressed on specific cells.
As a proof of concept, the researchers aimed to deliver AAVs to the mouse brain by targeting LY6A and LY6C1, proteins on the surface of cells that line blood vessels in the brain and make up part of the blood-brain barrier in mice.
In a test tube, Huang and her colleagues mixed a pooled library of AAV capsids with the proteins they wanted to target. They then looked for capsids that bound to the target proteins and identified patterns in the capsids' amino acid sequences. This allowed them to group similar capsids together.
Next, they tested representative capsids from top performing groups in mice to see which ones reached the brain. They identified hundreds of capsids that bound to LY6A or LY6C1 and delivered their cargo to the central nervous system in mice more efficiently than AAV9, a capsid that crosses the blood-brain barrier and is used in an FDA-approved gene therapy, but is inefficient at delivering to the brain.
"We're going back to first principles—rather than screening a random library in vivo, we focus on the cell type we want to engage and targeting specific proteins present on the surface of those cells," Deverman said. "The nice thing about this approach is that we find so many examples in a test tube that we can test in mice. That allows us to take a lot more shots on goal than with in vivo screening."
AAV possibilities
For the second round of capsid screening, the researchers added to the library 26 capsids that they and others had previously found bypassed the blood-brain barrier in animal models. The researchers found that 24 of these 26 capsids targeted either LY6A or LY6C1, suggesting that these two proteins are indeed the key targets of the AAVs that are identified through conventional library selections in mice.
These proteins, however, do not have human equivalents, but the researchers say that their screening approach will help scientists engineer AAVs to target human proteins much more efficiently.
Deverman, Huang, and others in the lab have already begun using their screening approach to home in on AAVs that bind to human proteins present on the blood-brain barrier. Over time, the team aims to use their experimental results to identify rules that help predict which proteins have the right characteristics to function as receptors for AAVs and guide the design of AAVs for new applications.
More information: Qin Huang et al, Targeting AAV vectors to the central nervous system by engineering capsid–receptor interactions that enable crossing of the blood–brain barrier, PLOS Biology (2023). DOI: 10.1371/journal.pbio.3002112
Journal information: PLoS Biology
Provided by Broad Institute of MIT and Harvard