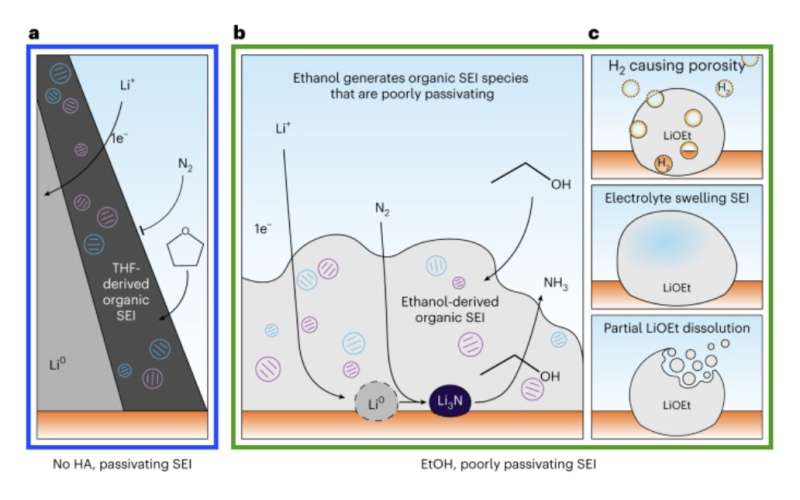
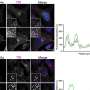
Panel A shows how the native SEI on Li metal is passivating to nitrogen gas, which means that no reactivity with Li metal is possible. Panel B shows that a proton donor like Ethanol will disrupt the SEI passivation and enable Li metal to react with nitrogen species. Panel C describes 3 potential mechanisms through which the proton donor can disrupt the SEI passivation. Credit: Steinberg et al.
Ammonia (NH3), the chemical compound made of nitrogen and hydrogen, currently has many valuable uses, for instance serving as a crop fertilizer, purifying agent, and refrigerant gas. In recent years, scientists have been exploring its potential as an energy carrier, to reduce global carbon emissions and help tackle global warming.
Ammonia is currently produced via the Haber-Bosch process, a carbon-producing industrial chemical reaction that entails the conversion of nitrogen and hydrogen into NH3. As this process is known to heavily contribute to global carbon emissions, electrifying the synthesis of ammonia would be very beneficial for our planet.
One of the most promising strategies for electrically synthesizing ammonia at ambient conditions is based on the use of lithium metal. However, some aspects of these process, including the properties and role of lithium's passivation layer, known as the solid electrolyte interphase (SEI), remain poorly understood.
Researchers at Massachusetts Institute of Technology (MIT), University of California- Los Angeles (UCLA), and the California Institute of Technology have recently carried out a study closely examining the reactivity of lithium and its SEI, as this could enhance lithium-based pathways to electrically synthesize ammonia. Their observations, published in Nature Energy, were collected using a state-of-the-art imaging method known as cryogenic transmission electron microscopy.
"Karthish Manthiram and I met first at Berkeley more than a decade ago, while he was a Ph.D. student and I was a graduate student," Yuzhang Li, one of the researchers who carried out the study, told Phys.org. "Karthish has been a long-term mentor to me, and we've kept in touch with each other's research. This collaboration opportunity naturally came about because of his efforts in lithium-mediated ammonia synthesis and my expertise in lithium metal batteries. These communities rarely talk to each other, but since Karthish and I often communicate, we started to learn more and more about key gaps in understanding of our respective fields."
The primary objective of the recent work by Li, Manthiram and their colleagues was to unveil the unique role that metallic lithium, particularly its SEI, plays in the electrochemical production of ammonia. To do this, the team examined the material's surface using an advanced, high-resolution microscopy technique that won the 2017 Nobel Prize in Chemistry.
"Since metallic Li is so reactive and unstable, it melts like butter under an electron beam typically used to study materials at atomic resolution (think of burning a hole in a leaf using a magnifying glass under the hot sun)," Li explained. "We instead cooled our samples in liquid nitrogen (very cold) and was able to image the atomic-scale surface of metallic lithium using a technique called cryogenic electron microscopy, a Nobel Prize winning tool that is also responsible for figuring out the molecular structure of COVID-19."
The analyses conducted by the researchers yielded some very interesting results, as they highlighted the crucial role of proton donors, such as ethanol, in governing the reactivity of lithium towards the fixation of nitrogen. They found that without ethanol or other proton donors, the SEI passivates metallic lithium, which prevents nitrogen reduction and thus the synthesis of ammonia.
"We discovered that the surface corrosion film of Li metal actually serves as a barrier to the reaction between Li metal and nitrogen gas (which is unexpected) and showed that this film had to be disrupted using a proton donor (e.g., alcohols like ethanol) to enable reactivity to form ammonia," Manthiram said. "This nanoscale understanding of the reaction pathway will guide future efforts to make this lithium-mediated approach to synthesizing ammonia more efficient and practical."
The findings gathered by this team of researchers could have crucial implications for the development of alternative and more environmentally friendly methods for synthesizing ammonia. By unveiling the crucial role of proton donors in lithium-based nitrogen reduction processes, this study could particularly promote the more efficient use of lithium in electrical ammonia synthesis.
"In our next works, we plan to study how the surface corrosion film properties change in different electrolyte chemistries and how they impact reactivity to produce ammonia," Li added.
More information: Katherine Steinberg et al, Imaging of nitrogen fixation at lithium solid electrolyte interphases via cryo-electron microscopy, Nature Energy (2022). DOI: 10.1038/s41560-022-01177-5
Journal information: Nature Energy
© 2023 Science X Network