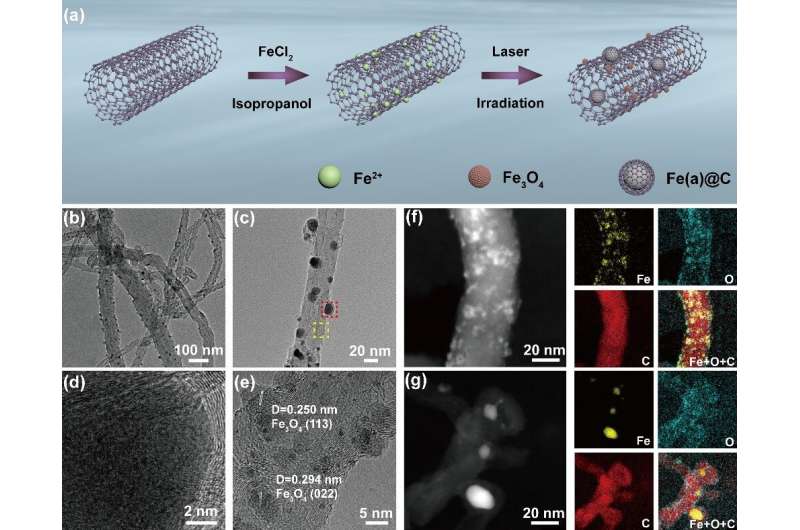
(a). Schematic illustration of the synthetic process of Fe(a)@C-Fe3O4/CNTs; (b). Low- and (c) high-magnification TEM images of Fe(a)@C-Fe3O4/CNTs. HRTEM images of Fe(a)@C (d) (from the red dashed box in c) and Fe3O4 NPs (e) (from the yellow dashed box in c); (f), (g) HAADF-STEM images and corresponding elemental mapping images of Fe3O4 and Fe(a)@C. Credit: Geng Jing
Urea (CO(NH2)2) has been applied both in agricultural and pharmaceutical field. The widely used Bosch-Meiser process has high energy consumption and CO2 emission. Therefore, it is imperative to explore energy-saving and economical routes for urea synthesis under mild conditions.
The electrosynthesis of urea with CO2 and NO3- under ambient conditions is an efficient way, but it is far from application. This is because the key step needs an efficient electrocatalyst enabling adsorption and activation of NO3- and CO2 to accomplish the C-N coupling.
Researchers from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences have now developed a liquid-phase laser irradiation route to fabricate symbiotic carbon encapsulated amorphous iron (Fe(a)@C) and iron oxide nanoparticles (Fe3O4 NPs) on carbon nanotubes (denoted as Fe(a)@C-Fe3O4/CNTs).
The as-fabricated Fe(a)@C-Fe3O4/CNTs contained two Fe-based active components, namely, Fe@C NPs with the particle sizes of 10~20 nm and Fe3O4 NPs with the particle sizes of 1~5 nm.
The presence of two different structural units in Fe(a)@C-Fe3O4/CNTs made it possible to synergistically electrocatalytic activate CO2 and NO3- to realize the C-N coupling for urea synthesis.
As expected, Fe(a)@C-Fe3O4/CNTs exhibited superior activity toward the electrocatalytic coupling of CO2 and NO3- for urea synthesis, affording a urea yield of 1341.3±112.6 μg h-1 mgcat-1 and a faradic efficiency of 16.5±6.1% at -0.65 V (vs. RHE) in 0.1 M KNO3 electrolyte.
Both experimental and theoretical results unveiled that Fe(a)@C was mainly responsible for the electrocatalytic reduction of NO3- to form *NH2 intermediates, while Fe3O4 was more beneficial for the electrocatalytic reduction of CO2 to form *CO intermediates.
The synergistically catalytic effect contributes to the excellent electrocatalytic performance of urea synthesis at ambient conditions.
The research was published in Angewandte Chemie International Edition.
More information: Jing Geng et al, Ambient Electrosynthesis of Urea with Nitrate and Carbon Dioxide over Iron‐Based Dual‐Sites, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202210958
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences