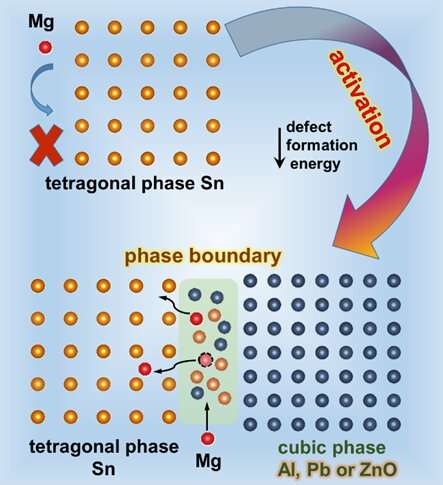
Through introducing the second phase, the increased phase boundary makes Mg easier to diffuse and enter into Sn, which effectively stimulates the electrochemical reactivity of Sn with Mg. Credit: Science China Press
Relatively arduous alloying reaction and sluggish diffusion kinetics of Sn-based anodes limit their practical applications in magnesium ion batteries (MIBs). To deal with these dilemmas, a general strategy was proposed to regulate the electrochemical reactivity and performance of Sn-based anodes for Mg storage through the introduction of the second phase and phase boundary.
The biphase Sn–Al, Sn–Pb and Sn–ZnO films were further fabricated via magnetron co-sputtering. Taking Sn–Al as an example, it has been revealed that the introduction of Al can effectively stimulate the electrochemical reaction of Sn with Mg in either nanoscale or bulk through combining experiments with density-functional theory calculations. Specially, the rolled Sn–Al electrode exhibits superior long-term stability over 5000 cycles.
Additionally, the Mg-storage mechanism of the Sn–Al electrode was investigated by operando X-ray diffraction. The Sn–Al anodes also demonstrate good compatibility with simple Mg-salt-based electrolytes like Mg(TFSI)2 in full cells. More importantly, it has been authenticated that the activation effect of second phase and phase boundary to Sn is also applicable to Pb and ZnO. These findings may provide a favorable reference for the development of alloy-type anodes for MIBs.
This study was led by Prof Zhonghua Zhang (School of Materials Science and Engineering, Shandong University). Results were published in Science China Chemistry.
More information: Meijia Song et al, Phase-boundary regulation boosting electrochemical reactivity of tin-based anodes for magnesium-ion batteries, Science China Chemistry (2022). DOI: 10.1007/s11426-022-1293-2
Provided by Science China Press