Surface effect of electrodes revealed by operando surface science methodology
Surface and interface play critical roles in energy storage devices, thus calling for in-situ/operando methods to probe the electrified surface/interface. However, the commonly used in-situ/operando characterization techniques such as X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray spectroscopy and topography, and nuclear magnetic resonance (NMR) are based on the structural, electronic and chemical information in bulk region of the electrodes or electrolytes.
Surface science methodology including electron spectroscopy and scanning probe microscopy can provide rich information about how reactions take place on the solid surfaces. But the applications of the sophisticated surface science methods in complicated electrochemical systems still remain less explored and more challenges. The main reasons are that the surface science methodology is commonly done in ultra-high vacuum (UHV) condition and over model structures with the open and well-defined surfaces.
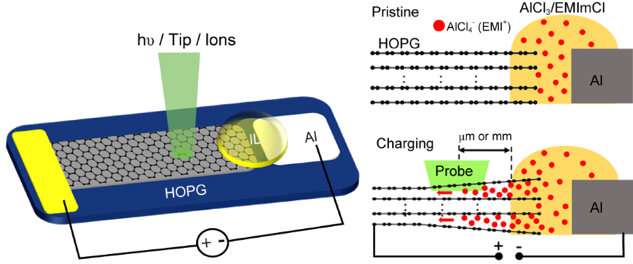
In a new research article (entitled "Operando Surface Science Methodology Reveals Surface Effect in Charge Storage Electrodes") published in the National Science Review, scientists at Dalian Institute of Chemical Physics (CAS) in Dalian, China propose a new strategy to apply operando surface science methods to explore the electrochemical process in the surface region of electrodes. Chao Wang and Qiang Fu et al. successfully carried out multiple operando surface science characterizations including Raman, XPS, AFM and SKPM over a planar Al/HOPG model battery. Intercalation of super-dense multilayer anions together with cations into the graphite electrode surface region has been directly visualized.
Based on UHV compatible ionic liquid (IL) electrolyte and well-defined electrodes, a planar Al/HOPG model battery which is composed of Al foil, HOPG flake and IL electrolyte in between has been designed for the following operando surface analysis. The model battery performs the same electrochemical behaviors as the real one. Furthermore, the diffusion length of the intercalated ions within HOPG model electrode can reach to millimeter. Thus, the electrochemical process can be directly probed on the open and clean electrode surface.
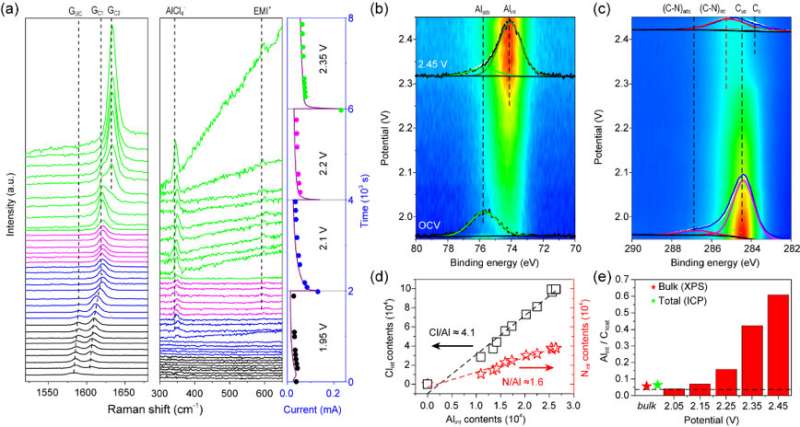
Operando Raman spectra have been acquired on the model battery. A stage-1 graphite intercalation compound (GIC) in the surface region is formed after being charged. In addition to the Raman signals of graphite, the co-intercalation of AlCl4- and EMI+ has also been discovered for the first time through operando Raman measurements. Subsequently, the model battery is further investigated by operando XPS. A set of XPS Al 2p and C 1s core-level signals are displayed. Co-intercalation of EMI+ has been further proved by operando XPS and its stoichiometric ratio with AlCl4 - is 4:5. The quantitative description of the charging mechanism in AIB has been proposed for the first time.
Notably, the intercalant ion concentrations in the surface region (Al/C 1:1.7) at the fully charged state (2.45 V) deduced from the operando XPS measurements are amazingly one order higher than the theoretical value (Al/C 1:24) dash line. Such results demonstrate the super-dense multilayer anions together with cations in the surface region. This distinct electrochemical process in the surface region can be further proved by quasi in-situ Raman, XPS, TOF-SIMS, and in-situ XRD/AFM measurements. The electrochemical behavior in the surface region and surface-dominant nanometer thickness graphite electrode can be described as the intercalation pseudocapacitance in contrast with the battery process in the bulk region. Based on the super-dense anion/cation intercalation mode in the surface region, the capacity can be doubled by using nanometer thickness graphite electrode in real coin-type AIB, which supports the operando characterization results based on the model devices.
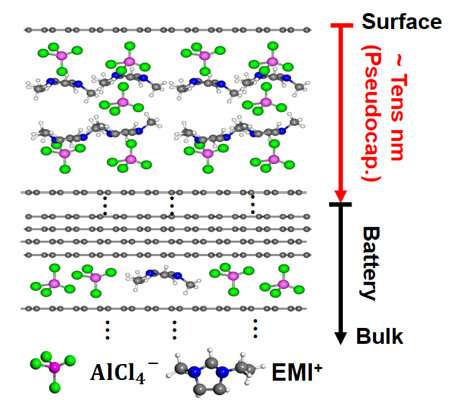
Based on the operando surface science analysis over a well-designed Al/HOPG model device, in depth and comprehensive charging mechanisms of AIB has been reached in this work. Particularly, an obvious surface effect has been discovered which can be used to improve the capacity. This work provides a new strategy of using operando surface science methodology to explore the surface/interface process in energy storage systems and highlight the critical role of the surface effect and surface science methodology in energy storage systems.
More information: Chao Wang et al, Operando surface science methodology reveals surface effect in charge storage electrodes, National Science Review (2020). DOI: 10.1093/nsr/nwaa289
Provided by Science China Press