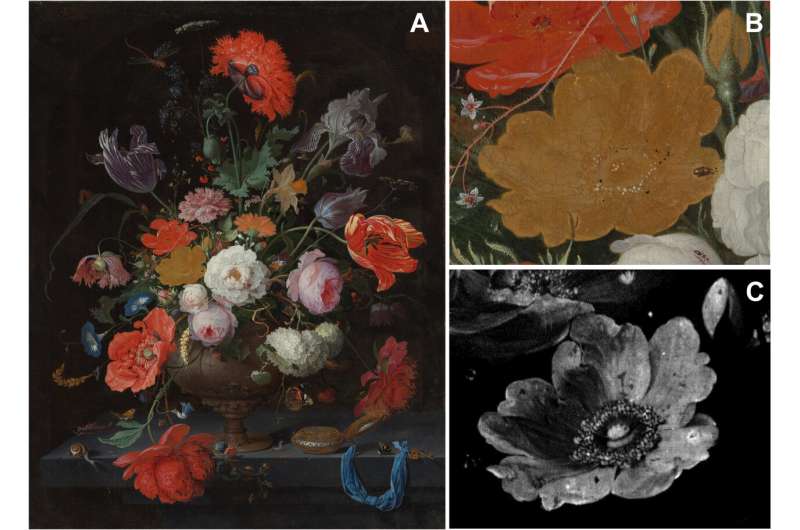
Pigment degradation in the yellow rose of Abraham Mignon’s Still Life with Flowers and a Watch. (A) Visual photograph of Still Life with Flowers and a Watch of Abraham Mignon (1640–1679), oil on canvas, dated c. 1660–1679, signed A. Mignon. Fc, from the collection of the Rijksmuseum (SK-A-268), (B) with a detail of the yellow rose (C) and the corresponding elemental distribution image of arsenic. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abn6344
A team of researchers from the University of Antwerp, the University of Amsterdam and Rijksmuseum has used chemical and optical imaging techniques to determine why a single flower in a famous painting faded while other flowers in the painting remained vibrant. Their work is published in the journal Science Advances.
Abraham Mignon was a 17th-century German painter renowned for his still life paintings, one of which was "Still Life with Flowers and a Watch"—it depicted a bouquet of bright and colorful flowers. Unfortunately, over time, one of the flowers—a yellow rose—has lost its brilliance and its once three-dimensional appearance. In this new effort, the researchers applied technology to the problem of understanding why the rose faded.
The researchers used several noninvasive techniques to study the flower, including X-ray fluorescence imaging, a technique that involves traditional X-rays to create images of objects. It is typically used to study sediments, minerals, fluids and rocks. The team also used X-ray powder diffraction, which images material by firing monochromatic X-rays at it along with a crystalline sample and indicating the ways they diffract. It is typically used to identify unknown crystalline materials.
The special way that Mignon layered his paints gave his work its unique 3D effects, so the researchers used these techniques to map the materials in the layers of the paint, such as lead, arsenic and calcium. The analyses showed that there were two crystals that had formed after the painting had been completed, both of which contained arsenic and lead due to a series of chemical reactions. The creation of the crystals led to the formation of arsenolite, which moved to different parts of the flower and reacted with other chemicals in the paint. And that led to the production of schultenite and mimetite, which led to color loss in the flower. In addition to losing color, the reactions also led to loss of the 3D effect, making the flower look flat and lifeless.
More information: Nouchka De Keyser et al, Reviving degraded colors of yellow flowers in 17th century still life paintings with macro- and microscale chemical imaging, Science Advances (2022). DOI: 10.1126/sciadv.abn6344
Journal information: Science Advances
© 2022 Science X Network