Mass-selected photoelectron matrices, slow photoelectron spectra (black solid lines) and photoionization yields (blue dotted lines). Credit: Lin Xiaoxiao
As a key precursor in the formation of new particles, sulfuric acid (H2SO4) plays an important role in the formation of aerosols and clouds in the atmosphere. Gas-phase sulfuric acid molecules can easily form molecular clusters at the beginning of nucleation through hydrogen bonding and other interactions. Therefore, study of the structure and spectroscopy of H2SO4 and tsulfuric acid–water clusters is of great significance for revealing the nucleation mechanism of new particles.
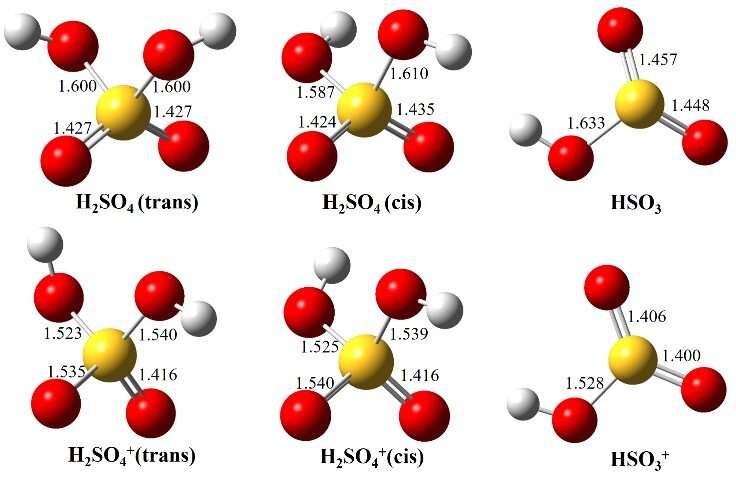
A team of researchers led by Prof. Zhang Weijun from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences (CAS), in collaboration with scientists from Synchrotron SOLEIL, France, has recently obtained the high-resolution slow photoelectron spectrum (SPES) of H2SO4 and observed three electronic states of H2SO4+, X2A, A2A and B2A, combined with high-level theoretical results.
For the first time, the "pure" high-resolution SPES of H2SO4 was found, without contaminations from other species in the continuous molecular beam.
The researchers investigated the vacuum ultraviolet photoionization of the sulfuric acid, using the state-of-the-art method of double imaging photoelectron photoion coincidence complemented with theoretical calculations.
The adiabatic ionization energy of H2SO4 towards X2A cationic ground state was in good agreement with high-accuracy theoretical data in the literature, according to the researchers.
When increasing photon energies, the H2SO4+ cation dissociated into HSO3+ and OH fragments. In addition, the enthalpies of formation for the species were determined by thermochemical cycle.
Optimized structures of H2SO4 (trans and cis conformers) and HSO3, as well as their individual cations, in the ground electronic states. Credit: Lin Xiaoxiao
This work provides fundamental data and measurement method for in-depth understanding of atmospheric nucleation mechanism.
The findings were published in Physical Chemistry Chemical Physics.
More information: Cuihong Zhang et al, Vacuum ultraviolet photochemistry of sulfuric acid vapor: a combined experimental and theoretical study, Physical Chemistry Chemical Physics (2021). DOI: 10.1039/D1CP05237C
Journal information: Physical Chemistry Chemical Physics
Provided by Chinese Academy of Sciences