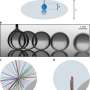
Figure 1. (a) Experimental setup. (b) Direct coalescence of two water drops in Leidenfrost state. (c) Consecutive bouncing of an ethanol droplet (transparent one) against a water droplet (tinted with methylene blue) during several seconds before coalescing. (d) Path of an acetonitrile droplet (tinted in blue) bouncing several times against a water drop. The snapshots show that the water droplet, initially transparent, turns bluish suddenly when the droplets coalesce. In (b)–(d), the elapsed time is indicated in seconds in each snapshot, with t=0s corresponding to the moment of coalescence [scale bars: (b) and (c) 10 mm, (d) 5 mm]. Credit: DOI: 10.1103/PhysRevLett.127.204501
A small team of researchers from Benemérita Universidad and Universidad de las Américas Puebla, in Mexico and Université de Poitiers, in France, has found a "triple Leidenfrost effect" in dissimilar drops in a hot pan. In their paper published in the journal Physical Review Letters, the group describes a type of "bouncing" they observed with different types of drops hovering over a hot surface.
Prior research has shown that the reason drops of water zip around in a hot pan, is because water at the bottom of the drops is vaporized—thus, the drops hover like air-hockey pucks. This phenomenon has come to be known as the Leidenfrost effect. In this new effort, the researchers have found another drop behavior associated with the Leidenfrost effect.
The work involved dropping two types of liquid onto a hot surface and then tilting the surface to force the drops to run into each other. They wanted to know if the two drops would merge. Instead they found that sometimes one of the drops would start bouncing off of the other.
Over several trials, the researchers tried several liquids: water, ethanol, methanol, isopropanol, acetone, hexane, chloroform, acetonitrile, toluene and formamide. They found that sometimes the two drops mixed right away, while sometimes they did not. After much trial and error, they found differences in properties of the two drops and their relative size to one another. Two drops of water, for example, because they have identical properties, merge right away. A drop of ethanol and a drop of water, on the other hand, have very different properties. When a small drop of ethanol was placed next to a large drop of water, the smaller drop bounced off of the larger drop several times before they merged.
An ethanol droplet bounces around a water droplet before eventually merging. The temperature of the concave metal surface is 250 C . Credit: F. Pacheco-Vázquez et al.
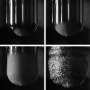
Ethylene glycol (clear) and chloroform (blue) have such different boiling points that the vapor layer between the droplets is visible when they collide.. Credit: F. Pacheco-Vázquez et al.
When droplets of ethylene glycol and chloroform merge, the chloroform droplet explodes because its boiling point (54 C) is so much lower than that of ethylene glycol (190 C). Credit: F. Pacheco-Vázquez et al.
The researchers found that the bouncing was due to the difference in boiling point and evaporation speeds of the two substances. They found that there was a Leidenfrost effect happening between the two drops—the hotter drop worked as a hot surface for the cooler drop; its vapor pushed the cooler drop away. They have labeled this phenomenon as a "triple Leidenfrost effect."
More information: F. Pacheco-Vázquez et al, Triple Leidenfrost Effect: Preventing Coalescence of Drops on a Hot Plate, Physical Review Letters (2021). DOI: 10.1103/PhysRevLett.127.204501 . Arxiv: arxiv.org/abs/2107.00438
Journal information: Physical Review Letters
© 2021 Science X Network
![Figure 1. (a) Experimental setup. (b) Direct coalescence of two water drops in Leidenfrost state. (c) Consecutive bouncing of an ethanol droplet (transparent one) against a water droplet (tinted with methylene blue) during several seconds before coalescing. (d) Path of an acetonitrile droplet (tinted in blue) bouncing several times against a water drop. The snapshots show that the water droplet, initially transparent, turns bluish suddenly when the droplets coalesce. In (b)–(d), the elapsed time is indicated in seconds in each snapshot, with t=0s corresponding to the moment of coalescence [scale bars: (b) and (c) 10 mm, (d) 5 mm]. Credit: DOI: 10.1103/PhysRevLett.127.204501 'Triple Leidenfrost effect' seen in dissimilar drops in a hot pan](https://scx1.b-cdn.net/csz/news/800a/2021/triple-leidenfrost-eff-1.jpg)