September 23, 2021 feature
Study unveils the minimum temperature for droplets levitating from smooth surfaces
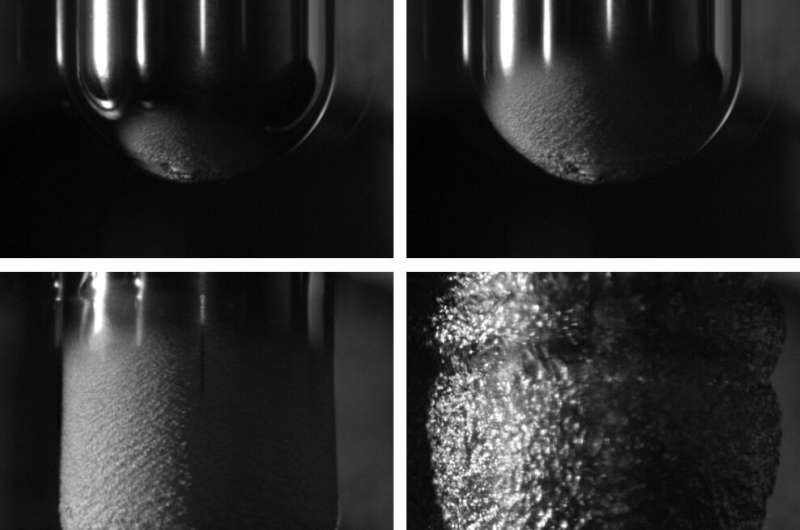
The Leidenfrost effect is a well-known physical phenomenon first discovered in 1756. It occurs when a liquid is in the proximity of a surface that is significantly warmer than its boiling point. This produces an insulating vapor layer that prevents the liquid from quickly boiling. Due to this effect, a droplet would hover over the surface instead of physically touching it.
While the Leidenfrost effect has been discovered centuries ago, the reported temperatures at which the vapor layer starts forming vary significantly from study to study. Many physicists worldwide have thus continued examining this phenomenon to better understand when and how it occurs.
Researchers at Emory University have recently demonstrated that Leidenfrost vapor layers can be sustained at far lower temperatures than those required for their formation. Their findings, published in Physical Review Letters, could have both theoretical and practical implications for several areas of physics.
"My lab has been working on the Leidenfrost effect for many years now," Justin C. Burton, one of the researchers who carried out the study, told Phys.org. "Our previous work focused on the interesting dynamics of levitated Leidenfrost drops, how they move, how they oscillate, etc. This was usually done at very high temperatures, where the thin vapor layer that exists between the drop and the hot surface is quite robust, even though the vapor layer is approximately the thickness of a human hair."
While the past studies conducted by Burton and his colleagues gathered interesting insight, a crucial open question still remained: what is the Leidenfrost temperature? In other words, the exact temperature required for the vapor layer to form on top of a surface and for it to be sustained over time remained unclear.
Physicists have not yet discovered with certainty how the vapor layer finally dissipates, yet they observed that its dissipation is accompanied by the liquid touching the solid surface and rapid, explosive boiling. In addition to informing physics research, providing an answer to these questions would also be valuable for several industries that utilize cooling hot objects and even for planetary sciences that explore phenomena such as phreatomagmatic eruptions.
"We set out to answer these questions using an electrical technique to precisely monitor the thickness of the vapor layer during formation, and as the hot material cooled down, all the way until the vapor layer spontaneously collapsed," Burton explained. "By adding a bit of salt to the water, the liquid acted as part of an electrical circuit, and the thin vapor layer acted as a capacitor. This allowed us to monitor the vapor layer in high-speed, milliseconds before and after the moment of collapse."
In addition to collecting several measurements of the vapor layer, Burton and his colleagues used high-speed video to examine the exact moment in which the layer collapses. Surprisingly, they found that while to form a vapor layer around a hot metallic object immersed in water, one needs to heat it up to ~240 degrees C, this same vapor layer can then remain stable as the object cools down to ~140 degrees C. In addition, the lower temperature at which the levitating droplets were sustained did not depend on salt concentration or the type of metal used in the experiment.
"I think the most notable finding of our work is that there seems to be a lower temperature to maintain the stability of Leidenfrost vapor layers, and that there is an 'upper temperature' for formation and a 'lower temperature' for failure," Burton said. "This is a very practical finding that will go beyond basic physics."
In the future, the results gathered by this team of researchers could inform research in a broad variety of fields. In fact, the physics of thin, lubricating liquid and gas layers is an ongoing topic of enquiry in many areas, from the study of friction to soft tissues, nanoscale cooling and microfluidics.
"We are currently conducting a series of numerical simulations to understand how the stability of the vapor layer disappears at the lower temperature," Burton added. "It's a very repeatable feature of the experiment, and thus it must be based on basic hydrodynamics. Like when a raindrop forms on a leaf and as it becomes larger, eventually it will fall off. This instability is caused by an excess of gravitational forces over surface tension forces, yet we currently don't know how the vapor layer became unstable in our experiment."
More information: Dana Harvey et al, Minimum Leidenfrost Temperature on Smooth Surfaces, Physical Review Letters (2021). DOI: 10.1103/PhysRevLett.127.104501
Journal information: Physical Review Letters
© 2021 Science X Network




















