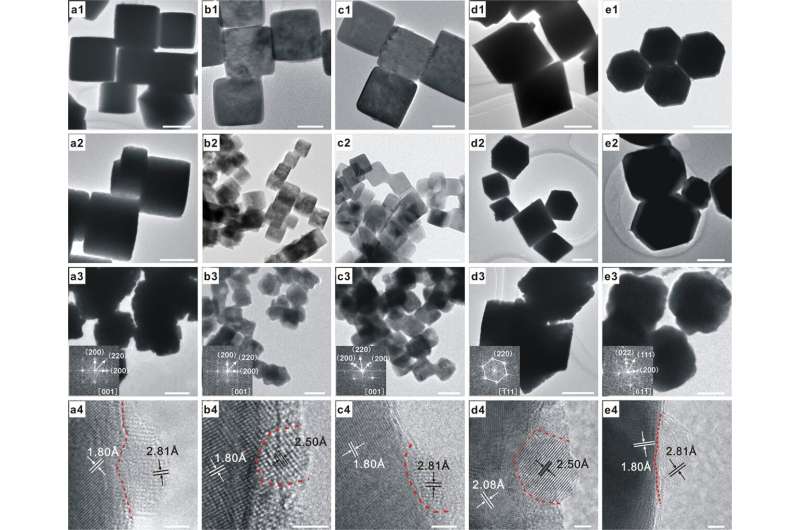
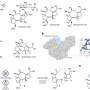
Fig. 1: Microscopic characterizations. The scale bars of (a1–a3), (d1–d3), and (e1–e3) correspond to 500 nm, that of b3 corresponds to 100 nm, those of b1, b2, c2, and c3 correspond to 50 nm, that of c1 corresponds to 20 nm, and those of (a4–e4) correspond to 2 nm. TEM images of as-synthesized a1 c-Cu2O-682, b1 c-Cu2O-109, c1 c-Cu2O-34, d1 o-Cu2O, and e1 d-Cu2O NCs. TEM images of as-synthesized a2 1%ZnO/c-Cu2O-682, b2 1%ZnO/c-Cu2O-109, c2 1%ZnO/c-Cu2O-34, d2 1%ZnO/o-Cu2O, and e2 1%ZnO/d-Cu2O catalysts. TEM and HRTEM images of as-synthesized (a3, a4) 1%ZnO/c-Cu-682, (b3, b4) 1%ZnO/c-Cu-109, (c3, c4) 1%ZnO/c-Cu-34, (d3, d4) 1%ZnO/o-Cu, and (e3, e4) 1%ZnO/d-Cu catalysts. Lattice fringes of 1.80, 2.08, 2.50, and 2.81 Å respectively correspond to the spacing of Cu{200}, Cu{111} (JCPDS card NO. 89-2838), hexagonal ZnO{101}, and ZnO{100} (JCPDS card NO 89-1397) crystal planes. Insets show corresponding electron diffraction patterns of TEM images. Credit: DOI: 10.1038/s41467-021-24621-8
The research team led by Prof. Huang Weixin and Assoc. Prof. Zhang Wenhua from University of Science and Technology of China of the Chinese Academy of Sciences, collaborating with Prof. Wang Ye from Xiamen University, investigated on the water gas shift (WGS) and CO hydrogenation reactions.
They observed the in situ reconstruction of the catalyst dependent on Cu structure and reaction atmosphere, and determined that CuCu(100)-hydroxylated ZnO interface and CuCu (611)Zn alloy were the active sites of Cu-ZnO catalyst for WGS reaction and CO hydrogenation to methanol reaction, respectively. This study was published in Nature Communications.
Since the introduction of the concept of "active site," identifying the active site structure of the catalyst has become the "Holy Grail" in the heterogeneous catalytic reaction. This kind of active site structure depends on the catalyzed chemical reaction.
Cu-ZnO-Al2O3 catalyst is widely used in commercial water gas shift (WGS, CO + H2O → CO2 + H2) and CO hydrogenation to methanol (CO + 2H2 → CH3OH), however, the catalytic active site structures of Cu-ZnO-Al2O3 catalyst in these two actions remain unclear.
In this study, the researchers prepared the well-structured ZnO / Cu catalyst via morphology maintaining reduction method based on the well-structured ZnO / Cu2O, and systematically studied the catalytic behavior of ZnO / Cu catalyst in WGS and CO hydrogenation to methanol with the help of in situ characterization technology and theoretical calculation. They found that in the water gas change reaction, ZnO/c-CuCu(100) catalyst showed the highest catalytic activity, and its catalytic performance was positively correlated with the number of Cu(I)CuCu(100)-ZnO interface sites.
Additionally, the researchers observed the in situ formation of CuZn alloy in the reaction of CO hydrogenation to methanol. The formation of CuZn alloy was positively correlated with the number of surface defect sites of Cu, and it was formed most easily at the surface defect site of c- CuCu(100) (Cu (611)). The methanol formation rate catalyzed by ZnO / CuCu(100) catalyst is positively correlated with the number of CuZn alloy sites. Combining these results with theoretical calculation, the researchers determined that CuCu (611)Zn alloy is the catalytic active site.
Prof. Huang has proposed the concept of "nanocrystalline model catalyst," and carried out researches on catalytic surface chemistry and determined catalyst active sites and catalytic mechanism under industrial catalytic reaction conditions. In former works, his group has studied the structured Cu2O / Cu nanocrystals, and a series of results were published in Angewandte Chemie (in 2011, 2014, and 2019) and Nature Communications.
More information: Zhenhua Zhang et al, The active sites of Cu–ZnO catalysts for water gas shift and CO hydrogenation reactions, Nature Communications (2021). DOI: 10.1038/s41467-021-24621-8
Journal information: Nature Communications , Angewandte Chemie
Provided by Chinese Academy of Sciences