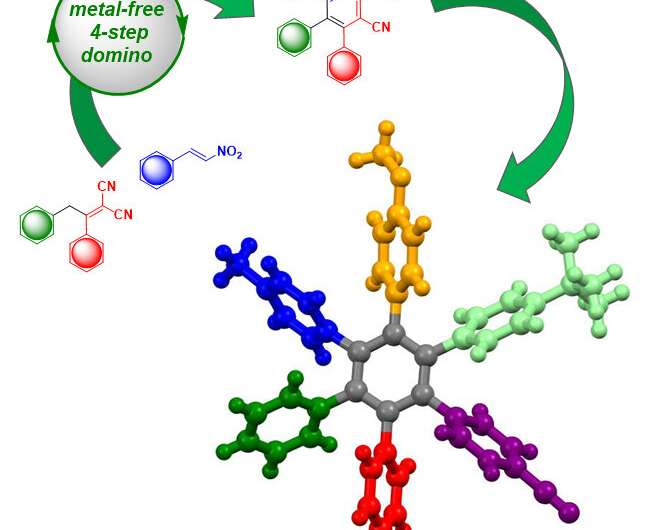
In a straightforward, four-step domino reaction, an initial compound can now be produced without the aid of – at times toxic – metals, and used to synthesize large quantities of asymmetrical HAB. Credit: FAU/Svetlana Tsogoeva
A team of researchers at the Department of Chemistry and Pharmacy at Friedrich-Alexander University Erlangen-Nürnberg (FAU) has successfully solved the problem of finding a straightforward, cost-effective process for producing hexaarylbenzene molecules with six different aromatic rings. These molecules are important functional materials. The results were published in the journal Angewandte Chemie.
Until now, it has been possible to use certain chemical procedures to produce simple, symmetrical hexaarylbenzene (HAB) molecules, in which the hydrogen atoms of the benzene are replaced by the same atomic groups. However, only very little HAB was produced in this way.
The team of researchers led by Prof. Dr. Svetlana Tsogeva and Prof. Dr. Norbert Jux, both professors of organic chemistry, has now developed a process which even allows asymmetrical HAB with six different aromatic rings around the benzene core to be produced simply and straightforwardly. In an efficient, four-step domino reaction, the researchers produced an initial compound without the aid of—at times toxic—metals, and used this compound to synthesize large quantities of asymmetrical HAB. Controls are carried out throughout the procedure to monitor which atom groups replace the hydrogen atoms of the benzene.
These currently unresearched HAB may be of use for developing innovative liquid crystal materials or for organic electronics.
More information: Benedikt W. Grau et al, Four‐step domino reaction enables fully controlled non‐statistical synthesis of hexaarylbenzene with six different aryl groups, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202104437
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by University of Erlangen-Nuremberg