August 6, 2020 report
A reaction using light and two transition-metal catalysts to make anilines

A team of researchers from the University of Manchester and pharmaceutical company AstraZeneca has developed a reaction that uses light and two transition-metal catalysts to make anilines. In their paper published in the journal Nature, the group describes their process and how it can be used. Valerie Schmidt, assistant professor of chemistry at the University of California San Diego, has published a News and Views piece in the same journal issue, outlining the importance of anilines and the work done by the team in the U.K.
Anilines are used in a wide range of products, from pharmaceuticals to dyes, agrochemicals, polymers and some electronic materials (which makes them very valuable). Looked at another way, they are benzene rings that have an attached nitrogen atom. They are typically made using toluene, benzene and xylenes, all of which are low-cost petrochemicals. And the method used is a nitration-reduction pathway. As Schmidt notes, they can also be made using aromatic substitutions. In this new effort, the researchers have developed a new reaction that might replace some of those currently used in production processes for creating anilines, giving companies a new option.
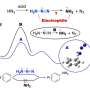
The method takes advantage of the fact that compounds containing nitrogen tend to react with carbonyl groups, resulting in C-N bonds. Such reactions result in the production of enamines which have a non-aromatic ring, which easily lend an electron when exposed to a light-activated catalyst. When the electron is lost, a reactive intermediate radical with an unpaired electron is created. The radical then interacts with a cobalt catalyst, which strips the hydrogen atoms from the non-aromatic rings, leaving an aromatic ring of aniline. The researchers call the process dehydrogenative amination.
The researchers tested their process by using it to make a host of anilines, many of which are commonly used in pharmaceuticals. They even went so far as to use the anilines they created to make several types of medicinal pills in common use. The researchers have not yet tested how well their approach can scale to manufacturing levels, however; thus, more work still needs to be done.
More information: Shashikant U. Dighe et al. A photochemical dehydrogenative strategy for aniline synthesis, Nature (2020). DOI: 10.1038/s41586-020-2539-7
Journal information: Nature
© 2020 Science X Network