Chemists prove the mechanism of direct amination from benzene
A team of chemists from Tomsk Polytechnic University discovered a mechanism of direct electrophilic amination and predicted the ways of its implementation. TPU scientists proved the most promising method for rapid and cost-effective production of aromatic amines. They are ones of the most sought-after organic synthesis products that are used in the manufacture of drugs, dyes, tires, and polymers. The study results were published in Chemistry Select.
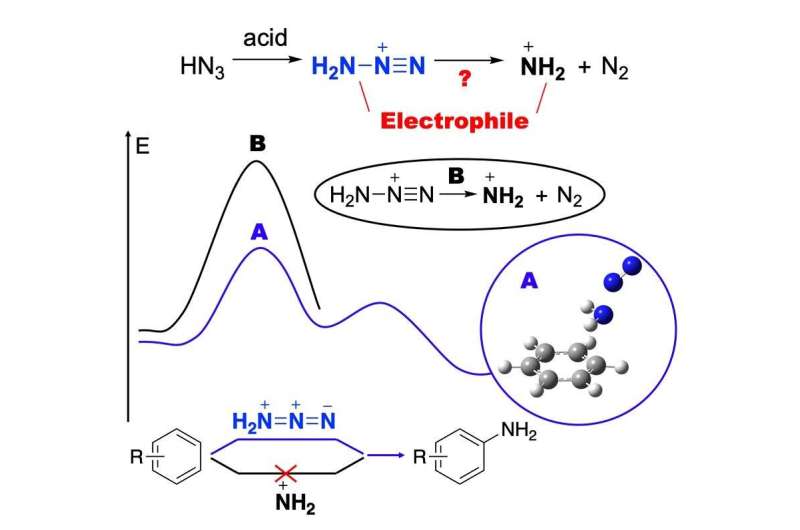
The study supervisor, Professor Viktor Filimonov from the TPU Kizhner Research Center says: "All existing methods for the production of aromatic amines involve several steps that are a number of chemical reactions taking a lot of time and reagents. Therefore, an electrophilic amination reaction i.e. the direct relation of the benzene nucleus and the amino group has always been the 'holy grail' of chemists. This reaction allows you to get the production in one-step route."
According to Filimonov, scientists from different countries are working in this area, including the Nobel Prize winner George Ola. Yet, nobody has achieved any results that could be widely applied in practice. In his opinion, one of the reasons for this is that the character of the reaction is understudied.
Over two years the scientists have managed to describe all the elementary stages of chemical transformations that occur during amination by using modern methods of quantum chemistry. The most important was to find a key particle called an intermediate that ensures the formation of the desired product. In this reaction, it turned out to be an aminodiazonium salt.
They hope that their study will give impetus and ensure chemists the ways of realizing their long-term dream of the direct synthesis of aniline from benzene.
TPU scientists proved theoretical calculations experimentally, and it became clear which starting materials can be used and under which conditions for the direct synthesis of aromatic amines.
"It is important to note that in the course of the study we have developed an absolutely new quantum chemical method for visualization or monitoring of changes of atomic and molecular orbitals, the transformation of their forms and distribution in space during chemical reactions. This method is universal and will be applied in both research and education of students," says Prof Filimonov.
More information: Ksenia S. Stankevich et al. Mechanism of Direct Electrophilic Aromatic Amination: an Electrophile is Found by Quantum-Chemical Study, ChemistrySelect (2019). DOI: 10.1002/slct.201803911
Provided by Tomsk Polytechnic University