This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Organic compounds show promise as cheaper alternatives to metal photocatalysts
Organic compounds that can be sustainably and affordably mass-produced show promise as replacements for expensive metal photocatalysts, according to a new report published July 17.
Scientists from the University of St Andrews demonstrated that a family of four organic compounds originally developed as emitters for organic light-emitting diodes performed as well, and in some cases, better, than archetypal photocatalysts.
The findings are reported in the journal Chem Catalysis.
Many traditional photocatalysts contain scarce, expensive and toxic metals
Professor Eli Zysman-Colman, an expert in optoelectronic materials from the School of Chemistry at St Andrews, said, "Photocatalysis, which involves using light and a dye to start the process of making compounds, has progressed at an incredible rate over the past 15 years.
"A central issue is that many archetypal photocatalysts contain metals like ruthenium and iridium. These are scarce, costly, and toxic. This makes them unattractive for late-stage use in the pharmaceutical and agrochemical industries.
"There's a real need for viable alternatives to these metal-based photocatalysts, particularly those that show strong reactivity, which is one of the research goals of my group."
Promising alternatives
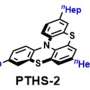
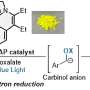
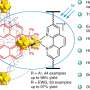
Zysman-Colman and pHd student Lea Hämmerling tested four boron- and nitrogen-containing multi-resonant thermally activated delayed fluorescence (MR-TADF) compounds as photocatalysts across a range of benchmark reactions.
Zysman-Colman said, "We identified that the intrinsic properties of these compounds—their strong absorption in the visible part of the spectrum, the relative insensitivity of their excited states to solvent polarity, and their electron-rich character—would make them very amenable as strongly photoreducing photocatalysts.
"We found that, when it came to their use in such reactions as pinacol coupling, dehalogenations and E/Z isomerizations, the MR-TADF compounds performed just as well or slightly better than several commonly used photocatalysts.
"This is significant: we've shown that this family of sustainable, organic materials performs competently across a wide range of transformation types.
"This should make them attractive photocatalysts for a broad range of applications, including for industries like pharma and agriculture that would prefer to have access to alternative photocatalysts to avoid trace metal contamination in the final product."
Zysman-Colman co-coordinates the PhotoReact Innovative Training Network comprising academia and industry to tackle the challenges associated with photocatalysis.
He adds, "Photocatalysis is a powerful synthetic tool that has transformed how we produce and access a wide range of chemicals, and offers the promise of a much more sustainable future for the production of these compounds."
More information: Lea Hämmerling et al, Building a photocatalyst library of MR-TADF compounds with tunable excited-state redox potentials, Chem Catalysis (2024). DOI: 10.1016/j.checat.2024.101061
Journal information: Chem Catalysis
Provided by University of St Andrews