This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemical chameleon reveals novel pathway for separating rare-earth metals
Researchers at the Department of Energy's Oak Ridge National Laboratory have found a chemical "chameleon" that could improve the process used to purify rare-earth metals used in clean energy, medical and national security applications.
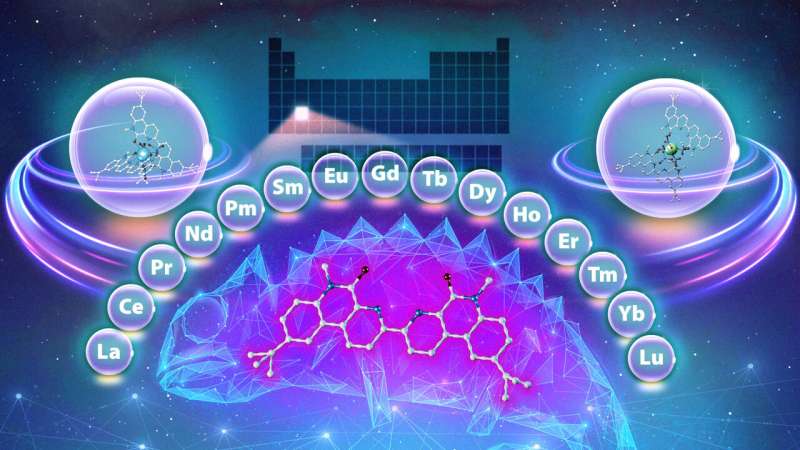
The study, performed in collaboration with Vanderbilt University, is the latest of many efforts by ORNL's Chemical Sciences Division to lower the barriers to accessing metals called lanthanides, which are used widely in diverse products and applications, from biomedical imaging to industrial chemical production to electronics. There are 15 lanthanides, and they, along with two other elements, are collectively known as the rare-earth metals.
The research is published in the Journal of the American Chemical Society.
Contrary to what their name implies, most rare-earth metals are not in fact rare; lanthanides are naturally occurring in mineral ore deposits, and many are as common in the environment as copper and lead. However, the powerful properties of the metals that cause them to be so widely used are only functional if an individual lanthanide is separated from the mixture of other metals in which it is present when mined. The metal of choice must be purified to a high level before it becomes useful in its intended application. The rarity lies in the difficulty of this process.
"It's a big challenge, because the lanthanide ions are very similar in their sizes and chemical properties," said Subhamay Pramanik, former ORNL postdoc and now radiochemist with ORNL's Nanomaterials Chemistry group. "They differ by only the slightest amount, so isolating pure individual lanthanides requires very precise separation science."
To isolate a select metal from rare-earth mineral solutions, scientists and industry use ligands—chemical compounds that selectively bind to a particular metal within the solution. These compounds are mixed into an organic solvent, then mixed with a water-based solution of the lanthanide mixture.
Like oil, organic solvents do not mix with water, so the layers separate. If the compound successfully grabs the target metal from the solution during mixing, it pulls the metal into the organic layer when the solvent and aqueous solution separate. Then the metal can be processed and further purified.
The best current industrial separation processes occur in stages, with lanthanides separated in a particular order—heavy to light or light to heavy. The process is time-consuming and costly, and it produces a lot of waste that is not always environmentally friendly.
Enter the chameleon. When studying an existing ligand similar to the compounds used in the aforementioned process, scientists discovered something unique: a ligand that behaves differently depending on the experimental conditions.
Like a chameleon changes its color to adapt to its environment, the compound changes its behavior when the environment around it changes, binding to different lanthanides depending on the acid concentration of the solution and the amount of time the ligand is allowed to interact with it. For example, if the environment is more acidic, the ligand preferentially binds to a heavier lanthanide.
"In typical separation systems, a ligand usually shows preference for either lighter or heavier lanthanides," said ORNL's Santa Jansone-Popova, who co-led the study. "We found you can use the same compound to perform multiple different separations, which is exciting and unique. And we identified the mechanisms by which it does it."
Using the same compound to separate multiple different lanthanides in the series could lower the number of steps needed in this common and costly process. Moreover, depending on the conditions, the ligand in this study could separate the heaviest, the lightest and mid-weight lanthanides—in any order.
Other ligands do not show this same behavior. However, until now, scientists did not know this one would either. The chameleon ligand looks similar to other well-established ligands, but it has completely different behavior. Now that it is known that such capabilities and systems exist for lanthanide-binding compounds, the ligand can be studied in more depth, and more compounds with similar behavior could be discovered.
"Just because the structure of a ligand looks very similar to another, it doesn't have to behave the same, and that understanding moves the needle and pushes the boundaries of what's known," said ORNL's Ilja Popovs, who co-led the study. "It has the potential to make the separation processes faster, cleaner and better—reducing the number of stages, providing better selectivity and purity, and leading to more environmentally friendly processes."
More information: Subhamay Pramanik et al, Tetradentate Ligand's Chameleon-Like Behavior Offers Recognition of Specific Lanthanides, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.4c07332
Journal information: Journal of the American Chemical Society
Provided by Oak Ridge National Laboratory