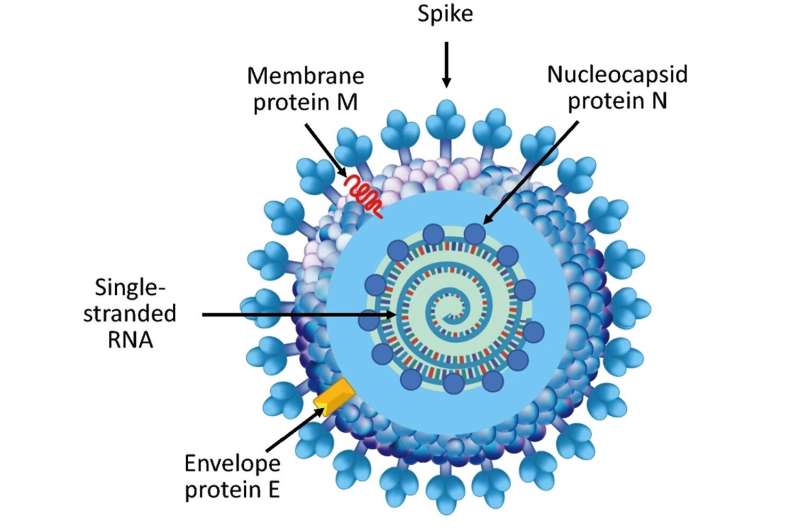
Cutaway illustration of a coronavirus particle. Research indicates that there are multiple ribonucleoprotein particles inside of each virion. Credit: Wikimedia Commons CC4.0
Of all the proteins of the SARS-CoV-2 virus (the pathogen that causes COVID-19), the spike protein is the one that gets the most attention. This attention is well-deserved—the spike protein is essential for latching on to cells and infecting a host, and the three FDA-authorized vaccines against COVID-19 focus on the spike protein. While the initial invasion of a virus into a host is certainly an important step in the viral lifecycle, once inside the cell, the virus must replicate to survive. And that's where the nucleocapsid protein, or N protein, comes into play.
The N protein is the most abundant protein of the SARS-CoV-2 virus. Its job is to package the viral genome into structures called ribonucleoprotein particles, which are then loaded into nascent virions that are released from an infected cell to wreak havoc on surrounding cells. Because of its essential role in viral assembly and replication, the N protein is a promising immunological target for anti-COVID-19 strategies.
"Given the unavoidable rise of SARS-CoV-2 variants, multi-pronged strategies that can prevent disease or limit the severity of COVID-19 are vital," said Peter Schuck, Ph.D., an NIBIB investigator in the Section of the Dynamics of Macromolecular Assembly. "The N protein is an essential player in viral assembly, but the exact way that this protein packages the SARS-CoV-2 genome is not known. If we could better understand this process, we might identify targets that would halt viral replication, providing an additional approach for COVID-19 vaccines or treatments," he said.
Schuck's research, recently published in iScience, investigates how the N protein interacts with oligonucleotides—short stretches of DNA and RNA—to demystify how the viral genome is packaged. Using biophysical methods, Schuck and his colleagues found out that when the N protein interacts with nucleotides of sufficient length, it adopts a shape that promotes interactions with other proteins. What's more, when the N protein binds with multiple copies of itself and long stretches of oligonucleotides, it can condense into highly concentrated droplets that are thought to ultimately enable the formation of ribonucleoprotein particles. Targeting interactions between the N protein and its binding partners might be a viable way to inhibit the viral replication of SARS-CoV-2, Schuck said.
"Our study is just a little piece of the puzzle posed by the SARS-CoV-2 viral lifecycle," Schuck said. "We now have some additional clues as to how the N protein initiates the assembly of ribonucleoprotein particles, an important—and potentially druggable—part of viral replication."
More information: Huaying Zhao et al, Energetic and structural features of SARS-CoV-2 N-protein co-assemblies with nucleic acids, iScience (2021). DOI: 10.1016/j.isci.2021.102523
Journal information: iScience
Provided by National Institutes of Health