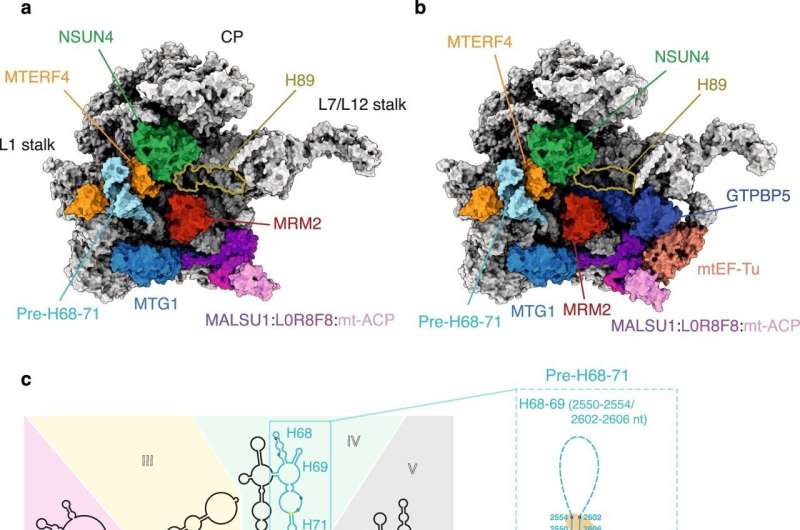
Fig. 1: Overview of the GTPBP5KO and the GTPBP5IP mt-LSU assembly intermediates and comparison with the mature mt-LSU. Credit: Nature Communications (2021). DOI: 10.1038/s41467-021-23617-8
In a study published in Nature Communications, researchers at Karolinska Institutet provide insight into the sequence of events leading to formation of functional mitoribosomes and sheds light on the mechanism of action of nine mitoribosome assembly factors involved in this process. The results may help yield novel opportunities for diagnosis and therapeutic intervention for mitochondrial diseases as well as cancer or diabetes.
Our cells' powerplants, mitochondria, convert nutrients into biochemical energy. The large protein complexes that perform this conversion work are made inside the mitochondrion by dedicated protein factories called mitoribosomes. Mutations affecting mitoribosomes and their biogenesis pathways lead to severe human mitochondrial diseases. However, the biogenesis pathways of human mitoribosomes remain poorly explored.
In the current study, published in the scientific journal Nature Communications, researchers from Karolinska Institutet resolved high-resolution cryogenic electron microscopy, called cryo-EM, to solve the high-resolution structures the human mitoribosomes trapped during their assembly process. Their study provides insight into the sequence of events leading to formation of functional mitoribosomes and sheds light on the mechanism of action of nine mitoribosome assembly factors involved in this process.
In addition, cryoEM data revealed an unexpected role for the translation elongation factor mtEF-Tu in mitoribosomal assembly. Specifically, mtEF-Tu apart from taking active role during protein synthesis, also coordinates binding of GTPBP5, one of the assembly factors during mitoribosome biogenesis.
Results provide molecular understanding of mitochondrial disease
Since dysfunction of mitochondrial protein synthesis is implied in a growing spectrum of clinical disorders, including cancer, diabetes, neurodegenerative diseases, and a large, clinically diverse group of disorders called mitochondrial diseases, the results may help yield novel opportunities for diagnosis and therapeutic intervention.
"Our data shed light on the mechanisms of action of many assembly factors that long-awaited structural characterization and reveal novel exciting evolutionary adaptations of the human mitochondrial translation system," says Joanna Rorbach, Assistant Professor at the Department of Medical Biochemistry and Biophysics at Karolinska Institutet, one of the senior authors of the study.
"This study greatly contributes to our understanding of the complex process of mitoribosome biogenesis and will help to better understand human diseases and development of new therapeutics," says Martin Hällberg, Senior Researcher at the Department of Cell and Molecular Biology, one of the senior authors of the study.
More information: Miriam Cipullo et al, Structural basis for late maturation steps of the human mitoribosomal large subunit, Nature Communications (2021). DOI: 10.1038/s41467-021-23617-8
Journal information: Nature Communications
Provided by Karolinska Institutet