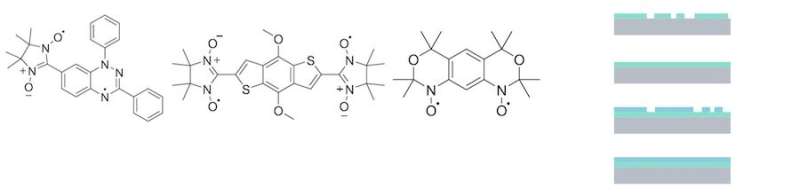
Left panel: The diradicals investigated in this work. Right panel: Sketch of different films (green colour) deposited on a substrate (grey colour). Credit: University of Tuebingen
Magnetism is a property of matter known by the humankind for several thousand years, long before these properties could be described in a theory. Classical magnets are metals or rare earth alloys, hard materials, such as refrigerator magnets.
Consider a class of materials carrying a magnetic moment, composed only of light elements, for example, carbon, nitrogen, and oxygen. This composition would allow researchers to have magnetic moments coupled to useful properties of organic materials, such as transparency, low-cost fabrication, and flexible chemical design. Indeed, this class of materials exists: it is the family of organic radicals. These radicals are organic molecules that carry one unpaired electron, giving rise to a permanent magnetic moment. Therefore, they are materials with permanent magnetic properties, i.e., their magnet moment is not due to an induction effect of an external magnetic field, such as in diamagnetism. Organic radicals are very promising materials for electronics and quantum technologies. The latest results on this class of materials by the Casu Lab team at the University of Tübingen's Chemistry Department have now been published in Chemistry of Materials.
In order to use these radicals in a device it is necessary to have them in film form, i.e., the molecules cover a substrate, forming a coating. In the Casu Lab team's research, they are deposited on a silicon wafer. The Tübingen scientists had started to think about this aspect ten years ago, when the German Research Foundation granted the Casu Lab the first project to prepare radical films in a controlled way using evaporation, pioneering the field of radical thin film processes. The research group has been successfully working on these materials since then.
Now the scientists have focused on systems that have more than one magnetic moment in the same molecule, that is, instead of a single unpaired electron, there are two unpaired electrons. They are called diradicals. Thus, there are two magnetic moments that can interact and influence each other, opening the avenue to new devices based on this interaction. The presence of two unpaired electrons makes these molecules very reactive, because the electrons have the tendency to pair. For a long time, it was thought that coating surfaces with this material using controlled evaporation would be practically impossible. The Casu Lab team tackled the problem by focusing on several diradicals based on the nitronyl nitroxide radical and the Blatter radical, and, recently, they were successful.
The Tübingen researchers have investigated the films using X-ray photoelectron spectroscopy, a technique based on the interaction of electromagnetic radiation with matter in the X-ray range. The measurements were performed in our lab in Tübingen, and at the BESSY synchrotron in Berlin.
The Casu Lab team describe their protocol and the recipe to evaporate diradicals in their paper published in Chemistry of Materials. From now on, anybody interested in new materials will be able to evaporate thin films of diradicals after reading the Tübingen researchers' paper.
More information: Tobias Junghoefer et al. Challenges in Controlled Thermal Deposition of Organic Diradicals, Chemistry of Materials (2021). DOI: 10.1021/acs.chemmater.0c03880
Journal information: Chemistry of Materials
Provided by University of Tuebingen