Catching radical molecules before they disappear
While in most molecules, each electron finds a partner to pair up with, some electrons in radical molecules are left alone and unpaired. This configuration grants radicals with some unusual and interesting properties, which disappear as soon as the radicals react or interact with other molecules. It has been difficult to generate relatively stable radicals, because they react and change in the blink of an eye, but researchers from the Center for Self-Assembly and Complexity, within the Institute for Basic Science (IBS, South Korea) succeeded in synthesizing four new kinds of stabilized radicals.
Unlike other molecules, some radicals have aligned spins, which confers them ferromagnetic properties, meaning that they can be attracted by a magnetic field. Due to these peculiar properties, radicals are likely to find applications in various fields, such as rechargeable batteries, molecular spintronics, and molecular magnetism.
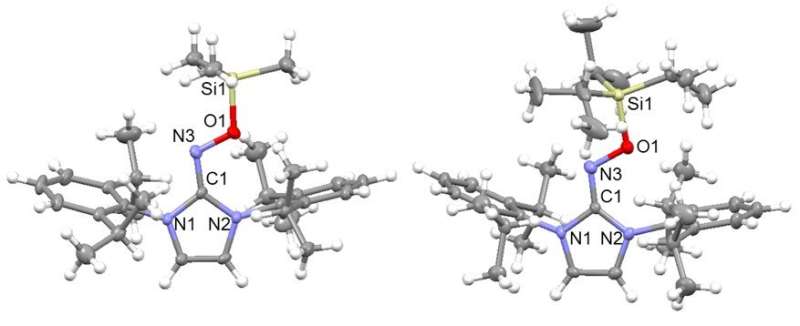
IBS scientists developed a strategy to stabilize oxime radicals, using N-heterocyclic carbenes (NHCs), as the latter can share their electrons to stabilize the unpaired electrons of the radicals. This result is particularly interesting as organic radicals are known to be very difficult to synthesize because they are more unstable than metal-containing radicals.
The radical structures were confirmed by single crystal X-ray diffraction analysis at Pohang Accelerator Laboratory and their properties were verified by electron paramagnetic resonance. The experimental results agreed well with the density functional theory.
The same research group has also recently stabilized triazenyl radicals and used them as cathode materials for rechargeable lithium ion batteries. In the future, the researchers are taking up the challenge of producing more radical chemicals that have yet to be synthesized.
More information: Youngsuk Kim et al. Oxime Ether Radical Cations Stabilized by N-Heterocyclic Carbenes, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201710530
Journal information: Angewandte Chemie International Edition
Provided by Institute for Basic Science