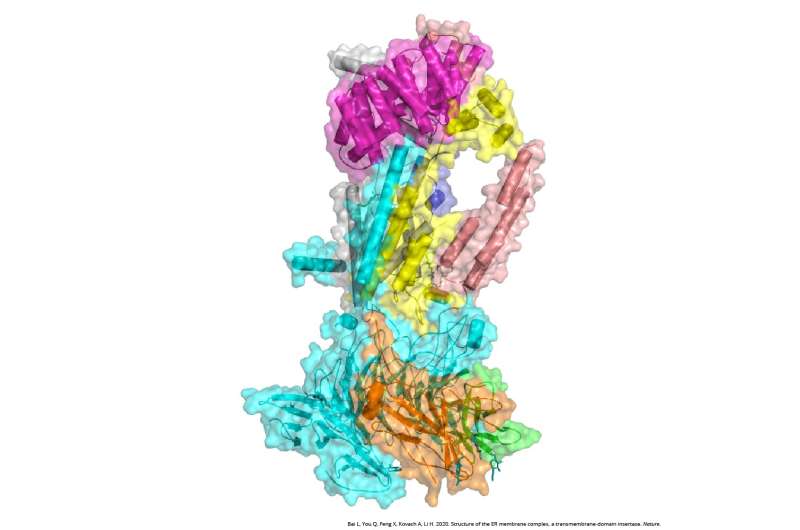
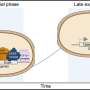
Cryo-EM structure of the endoplasmic reticulum membrane complex (EMC). Credit: Dr. Huilin Li, Van Andel Institute
Van Andel Institute scientists have revealed the first known atomic structure of a "molecular machine" responsible for installing critical signaling proteins into cellular membranes.
The findings, published today in Nature, shed new light on how this process works, and lay the foundation for potential future therapies for diseases like cancer, Alzheimer's and cystic fibrosis.
"Determining precisely how proteins are assembled and function is central to understanding how the body works on the most basic level," said VAI Professor Huilin Li, Ph.D., leader of the Institute's Structural Biology Program and the study's senior author. "Our findings provide a map for future studies that one day could be translated into ways to combat disease."
Proteins are the molecular workhorses of the body, responsible for carrying out nearly every biological function. Roughly one-third of proteins are membrane proteins, whose jobs include relaying information to and from cells, and transporting ions and molecules through the cell membrane, among other vital tasks. These important roles make them popular marks for therapy—more than half of the medications on the market target membrane proteins as a way to treat disease.
The endoplasmic reticulum membrane complex, or EMC, is embedded in the endoplasmic reticulum, a system of membranes within cells that plays a part in the creation, editing and transport of proteins. When the EMC's machinery breaks down, protein production can go awry, resulting in misshapen proteins that cannot do their jobs properly. For example, problems with the EMC are directly associated with cystic fibrosis, a genetic lung disease caused by improper assembly of a protein called CFTRDF508.
Using the Institute's powerful cryo-electron microscopes (cryo-EM), Li and his colleagues visualized the EMC of a common yeast strain. Yeast are commonly used models in biology because they have many of the same molecular systems as humans but are much simpler to study.
They found that the yeast EMC is larger than previously thought, containing eight protein subunits instead of six. Additionally, the core of the EMC resembles a protein that performs a similar function as the EMC in bacteria, suggesting that the EMC in eukaryotic organisms like mammals may have arisen from bacterial systems.
Collectively, the findings shed light on how membrane proteins become established in membranes and how they change from their unstructured form to their functional form once there—processes that have not been well understood until now.
More information: Structure of the ER membrane complex, a transmembrane-domain insertase, Nature (2020). DOI: 10.1038/s41586-020-2389-3 , www.nature.com/articles/s41586-020-2389-3
Journal information: Nature
Provided by Van Andel Research Institute