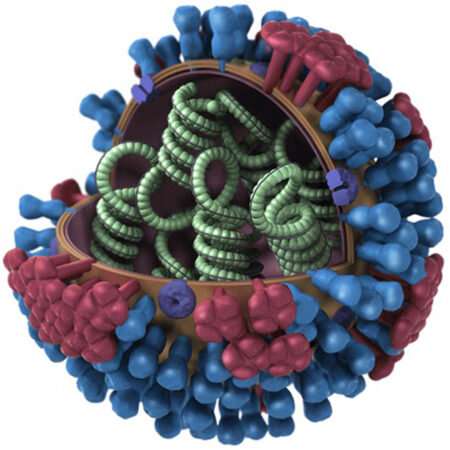
The influenza virus RNA-nucleoprotein complexes are shown in green. Credit: CDC/Dan Higgins
The viral genome of influenza A—the primary cause of seasonal flu epidemics—is composed of eight RNA segments that are each encapsulated by many copies of viral nucleoprotein (NP).
Evidence suggests that a host cell factor called UAP56, which has critical roles in RNA processing and export from the nucleus, facilitates encapsulation of viral RNA by NP, but the mechanism is not clear.
Yi Ren, Ph.D., and colleagues have now characterized the interaction between NP and UAP56 using recombinant proteins and mass spectrometry. They demonstrated that UAP56 features two NP binding sites. One of the sites, which was previously unknown, is in the N-terminal extension of UAP56 and recognizes the RNA-binding region of NP.
Their report in Biochemical and Biophysical Research Communications reveals the molecular basis for how UAP56 acts on RNA-free NP. The findings provide new insights into NP-mediated influenza genome packaging, which is critical for the virus life cycle and its ability to cause infection.
More information: Andrew K. Morris et al. Cellular mRNA export factor UAP56 recognizes nucleic acid binding site of influenza virus NP protein, Biochemical and Biophysical Research Communications (2020). DOI: 10.1016/j.bbrc.2020.02.059
Journal information: Biochemical and Biophysical Research Communications
Provided by Vanderbilt University