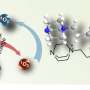
High steady-state singlet oxygen production, [1O2]ss, during one hour irradiation of air-saturated solutions of pyruvic acid in water. Credit: Marcelo I. Guzman
Researchers at the University of Kentucky found that when aqueous solutions with pyruvic acid, a 2-oxocarboxylic acid, were exposed to light, the generated triplet excited state could efficiently convert dissolved molecular oxygen into highly singlet oxygen. This finding is expected to contribute to areas such as environmental, life, and medical sciences in the future.
A research group comprising Associate Professor Marcelo Guzman and graduate student Alexis Eugene (completed Ph.D. program in 2019), from the Department of Chemistry at the University of Kentucky, found that when solutions of pyruvic acid in water were exposed to light in the solar spectrum, the photogenerated triplet excited sate could react with dissolved oxygen from dissolved air and generate highly reactive singlet oxygen. They also found that the concentration of singlet oxygen produced could be equivalent and even exceed the levels generated in fog and cloud waters by unidentified chromophores. Singlet oxygen is highly reactive oxygen species used in photodynamic therapy (e.g., treatment of microbial cells and cancer with light) and preparative organic chemistry, among multiple applications.
This finding is expected to contribute to areas such as environmental science and engineering for the processing pollutants in the atmosphere, life sciences by inducing chemical reactions of synthetic importance, and for future application of medical care in the detection, imaging and diagnosing, and treatment of carcinogenic cells.
The outcomes of this research were published in the online version of Environmental Science and Technology, a journal from the American Chemical Society on September 24.
More information: Alexis J. Eugene et al. Production of Singlet Oxygen (1O2) during the Photochemistry of Aqueous Pyruvic Acid: The Effects of pH and Photon Flux under Steady-State O2(aq) Concentration, Environmental Science & Technology (2019). DOI: 10.1021/acs.est.9b03742
Journal information: Environmental Science and Technology , Environmental Science & Technology
Provided by University of Kentucky
![High steady-state singlet oxygen production, [1O2]ss, during one hour irradiation of air-saturated solutions of pyruvic acid in water. Credit: Marcelo I. Guzman Converting Absorbed Photons by 2-Oxocarboxylic Acids into Highly Reactive Singlet Oxygen](https://scx1.b-cdn.net/csz/news/800a/2019/convertingab.jpg)